Therapeutic agent for intranasal administration and method of making and using same
a technology of intranasal administration and therapeutic agents, which is applied in the field of therapeutic agents, can solve the problems of parasympathetic hyperactivity, nasal congestion and drainage, and poor elucidation of nar, and achieves the effects of improving therapeutic response, rapid onset of action, and quick and/or fast onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
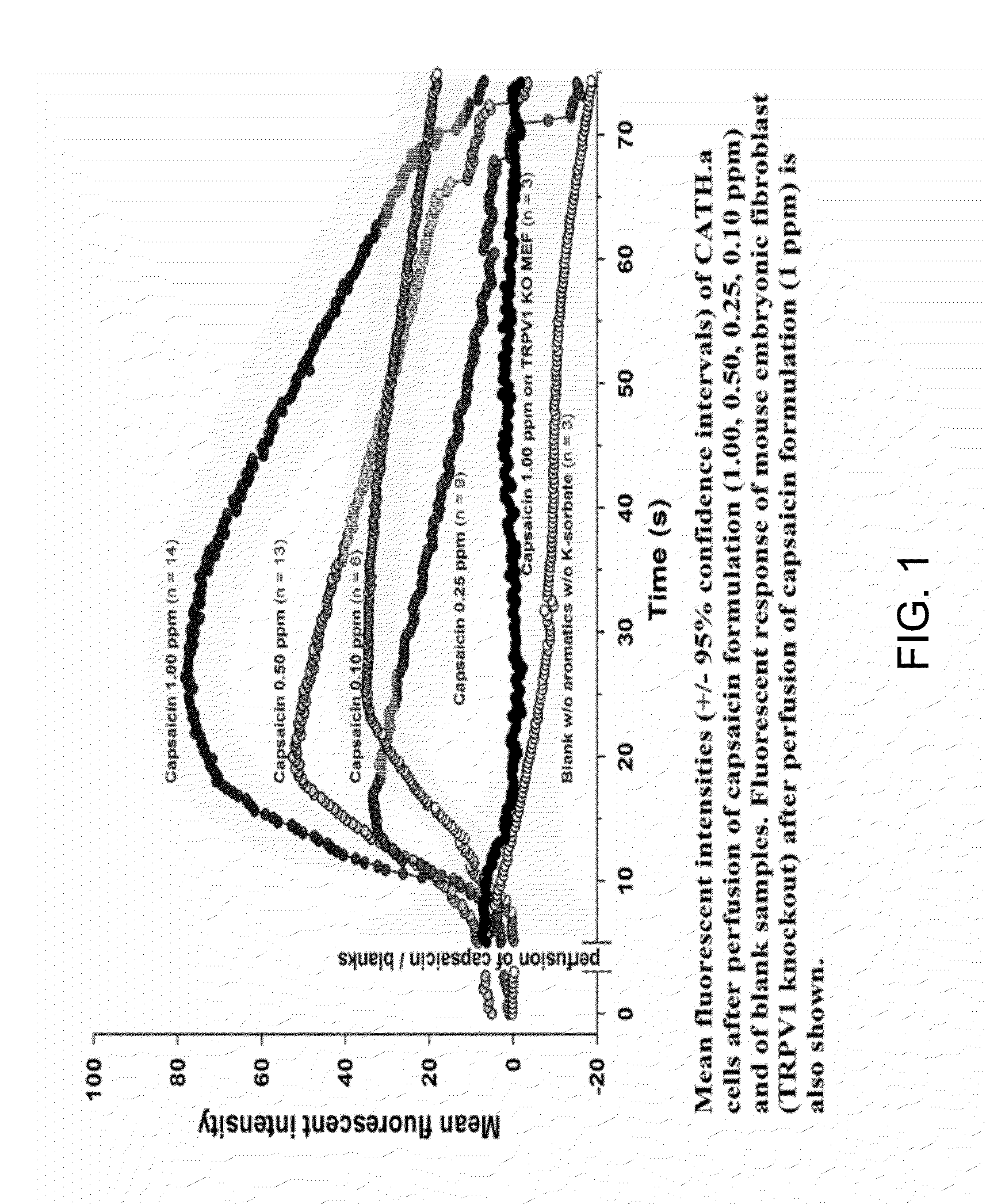
[0056]It is hypothesized that capsicum, comprising capsaicinoids, including capsaicin, and possibly other ingredients in Applicant's composition, used for relief of rhinitis and allergic symptoms act via activation of TRP-family receptors. Applicant has developed an in vitro fluorometric assay to measure receptor activity in the presence of various concentrations of capsaicin / capsaicinoids. The activation of the receptors in the in vitro assay correlates with Applicant's demonstrated symptom relief as evidenced in the examples below. Specifically, the goal of the in vitro assay is to quantitatively measure TRPV activation to determine the dose response of airway component cells to Applicant's compositions comprising capsaicin / capsaicinoids. To this end, Applicant developed a new assay for transient receptor protein vanilloid (“TRPV”) activation in cells. The assay is fluorometric in nature, relying upon a calcium-binding fluor, Fura-4. (available from Invitrogen Life Sciences and ot...
example 2
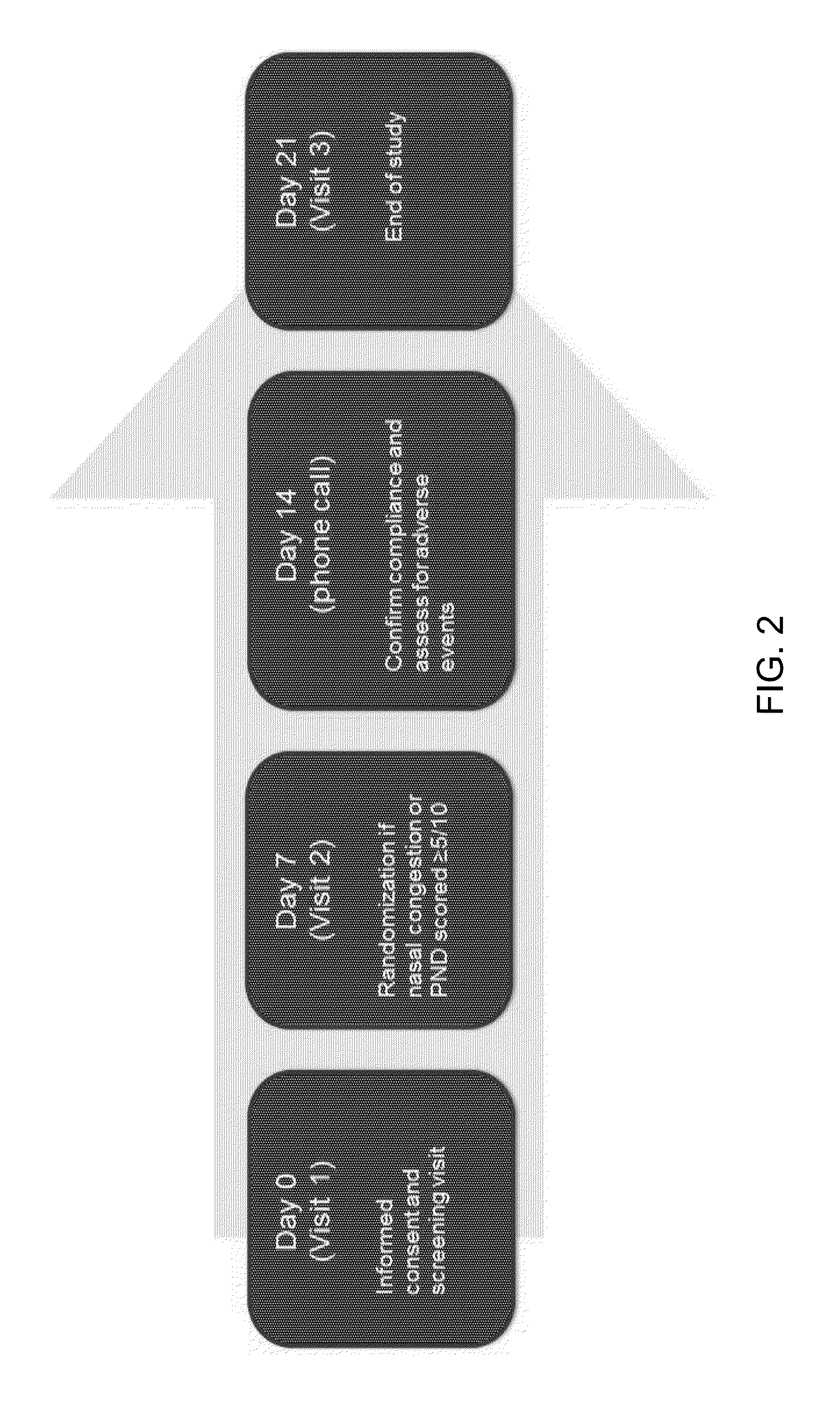
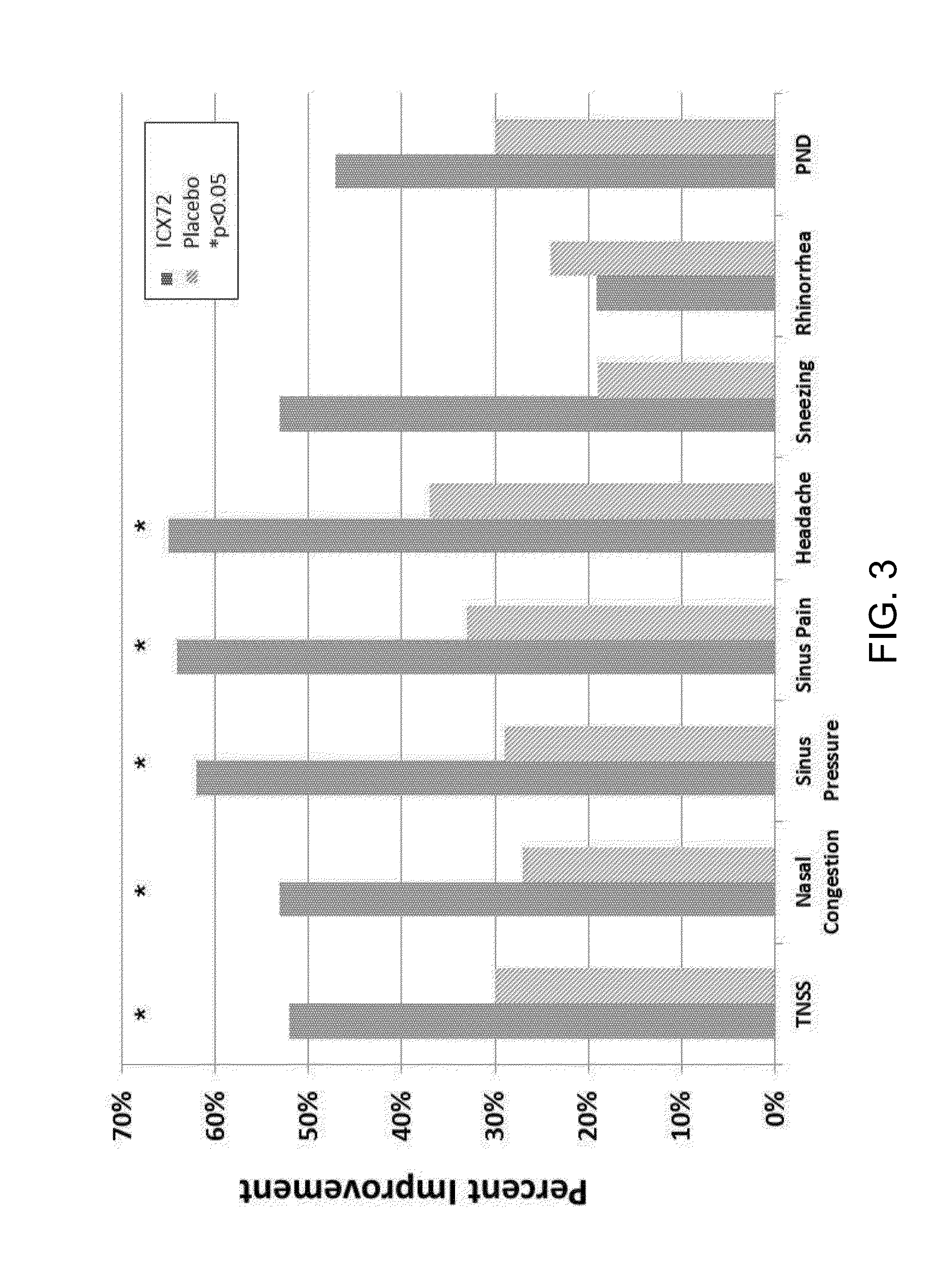
[0060]A clinical study was performed to assess the efficacy and safety of a newly formulated intranasally administered capsicum composition (“ICX”) used continuously over a two week treatment period in chronic rhinitis subjects who have a significant component of NAR. The ICX formulation comprises from about 0.00060% to about 0.010% w / w, or comprising about 0.1 to about 2.0 ppm total capsaicinoids, about 0.0020% to about 0.0080% w / w, resulting in about 0.3 to about 1.5 ppm total capsaicinoids, or preferably about 0.0030% to about 0.0080% w / w, resulting in about 0.5 to about 1.5 ppm total capsaicinoids. The ICX formulation further comprises rosemary extract at a concentration of about 0.02% to about 0.25% w / w, eucalyptol at a concentration of about 0.07 to about 0.15%, vegetable glycerin at a concentration of about 3.5% to about 5.0% w / w, ascorbic acid at a concentration of about 0.10% to about 0.90% w / w and sea salt at a concentration of about 0.40% to about 1.2% w / w. One embodiment...
example 3
[0097]One example of the present invention relates to a headache relief composition. The headache relief composition provides relief of symptoms such as migraines, general headaches, tension, chronic and occasional headaches, and dizziness, and visual distortions associated with headaches. The headache relief composition comprises capsicum comprising capsaicin, dihydrocapsaicin, and nordihydrocapsaicin, feverfew extract, eucalyptol, peppermint oil, rosemary extract, vegetable glycerin, ascorbic acid, citric acid, and sea salt. An embodiment may comprise potassium sorbate at a concentration of about 0.05% to about 0.30% w / w.
[0098]The feverfew extract relieves and prevents headache symptoms. The eucalyptol is used to ameliorate the sensory experience and soothe the nasal mucosa. The peppermint oil relieves and helps prevent headache symptoms. The rosemary extract is an anti-microbial agent and a natural preservative which protects and stabilizes the headache relief composition. In a s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com