Inorganic oxides for co2 capture from exhaust systems
a technology of inorganic oxides and exhaust systems, applied in the direction of machines/engines, separation processes, mechanical equipment, etc., can solve the problems of inability to meet the requirements of operation temperature, and inability to operate at 850° c,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
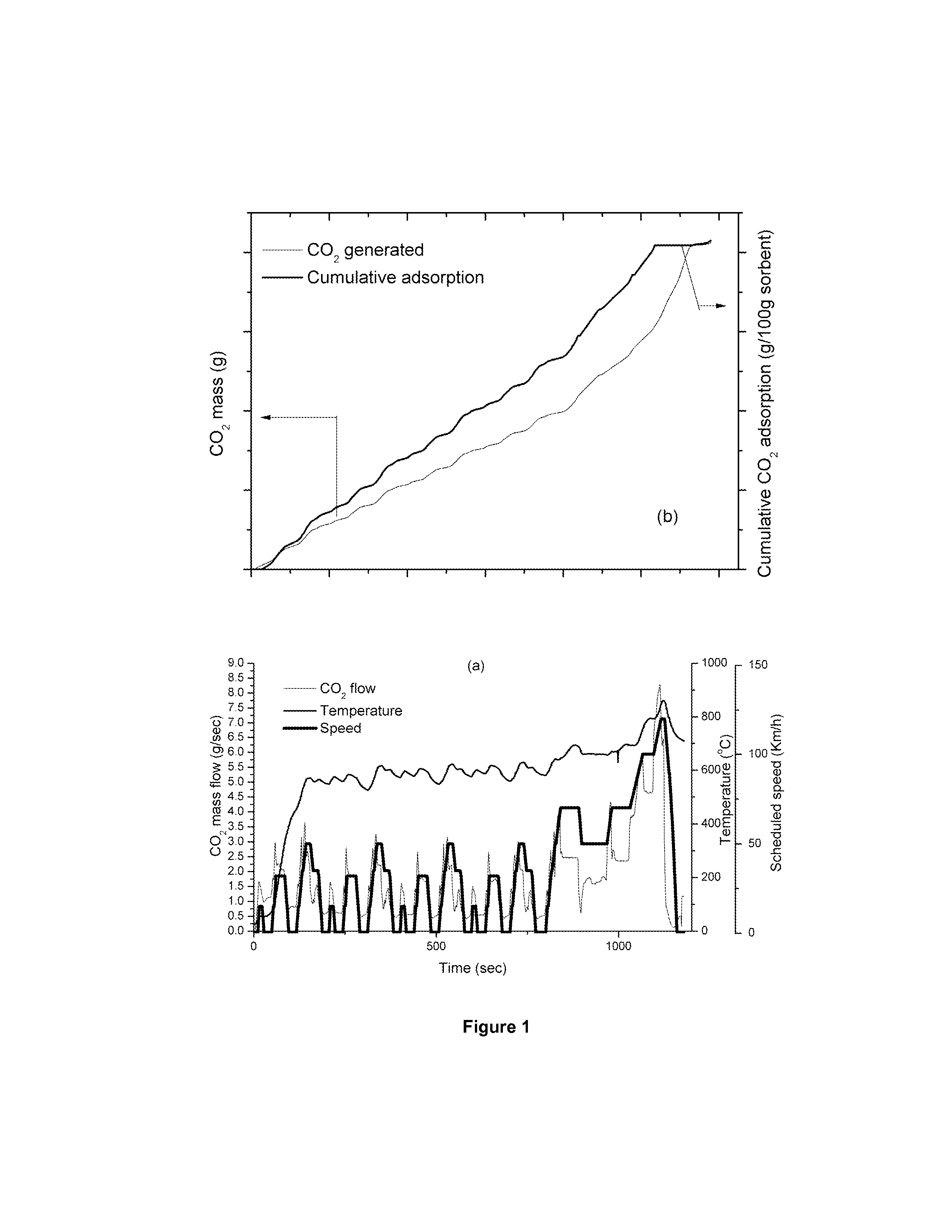
[0048]If we consider a conventional gasoline passenger car on the NEDC as shown in FIG. 1a the temperatures recorded at the engine-out position, T1. These temperatures and the amount of CO2 evolved increase as the speed increases. This indicates that lithium zirconate may be an efficient sorbent in the case of lower speeds, higher speeds leading to higher temperatures of the exhaust gas streams allowing for on board desorption. The data represented in FIG. 1b indicates the CO2 adsorption capacity of 100 g sorbent placed at position T1 over the drive cycle shown in FIG. 1a. This confirms the efficiency of lithium zirconate to remove CO2 over these operating parameters. Above these temperatures almost the entire amount of CO2 adsorbed is released and hence can be desorbed.
[0049]To remove the target level of 10% of the CO2 from the exhaust stream over the NEDC (206.2 g) then 732 g of sorbent will be required.
[0050]If the same lithium zirconate sorbent is placed in a position T2...
example 2
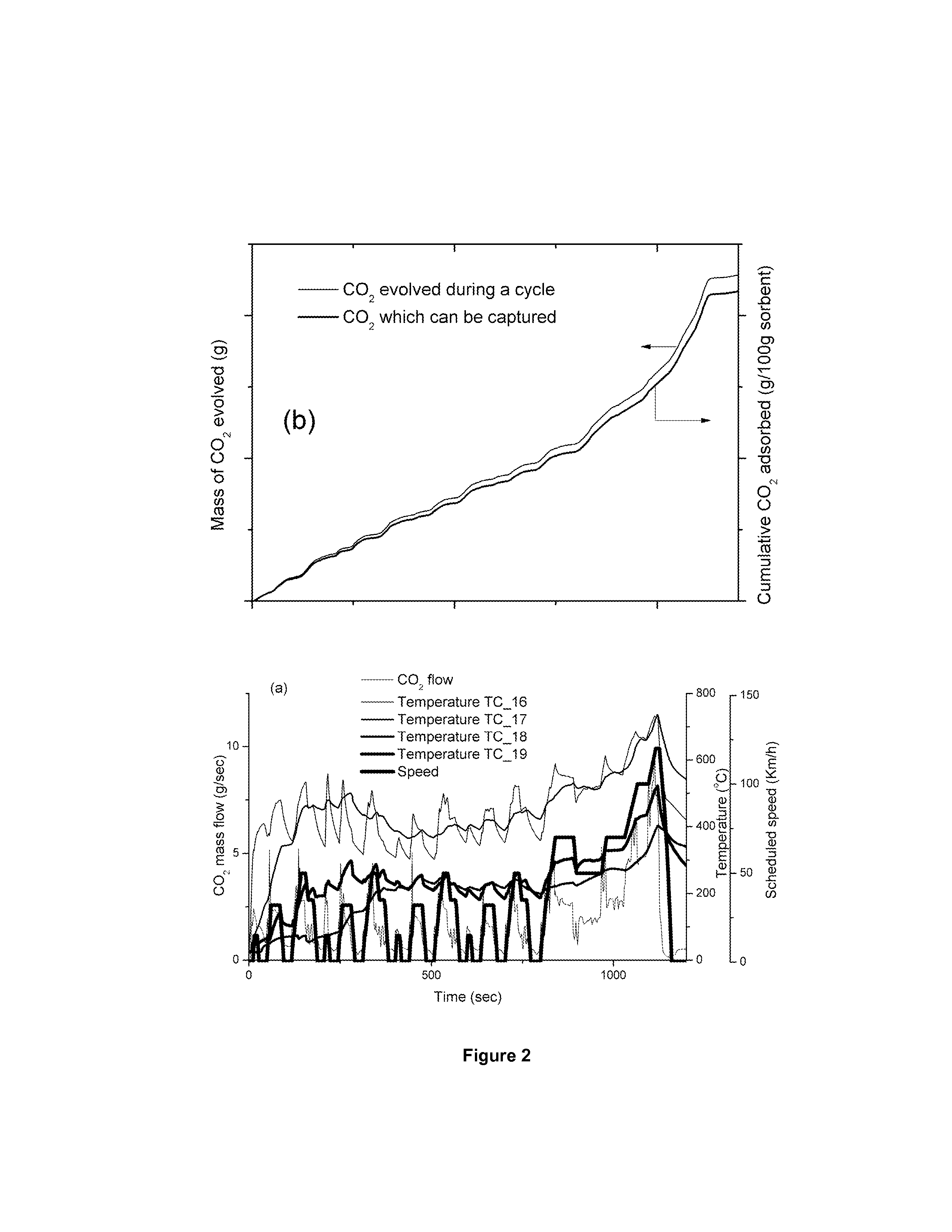
[0051]In the case of a lean burn combustion mode gasoline engine the temperatures of the exhaust gas stream are lower than in the previous case and the positioning of the sorbent can be tailored to increase its efficiency for CO2 removal. Thus, as it can be observed from FIG. 2a and tables 2 and 3, that positioning of the sorbent in points TC_18 (post NOx reduction catalyst) or TC_19 (immediately pre NOx reduction catalyst) will not be possible for lithium zirconates, and give low capacities for hydrotalcites, however positions TC_16 (engine-out) and TC_17 (post 3-way catalyst) will be suitable for both systems, but particularly good for hydrotalcites.
[0052]To capture the target level of 10% of the released CO2 over the drive cycle (228 g), to minimise the weight of sorbent, the most efficient use would be the Zr-hydrotalcite at position TC_17, for which only 180 g would be required, however this would assume a method of onboard storage of CO2 in separate system to...
example 3
Diesel
[0053]As suggested by FIG. 3a, in the case of diesel internal combustion engines, there is a relatively low temperature adsorption activity, this excludes the use of higher temperature sorbents such as the lithium zirconates, however is suitable for hydrotalcite type materials for CO2 removal from the exhaust gas streams.
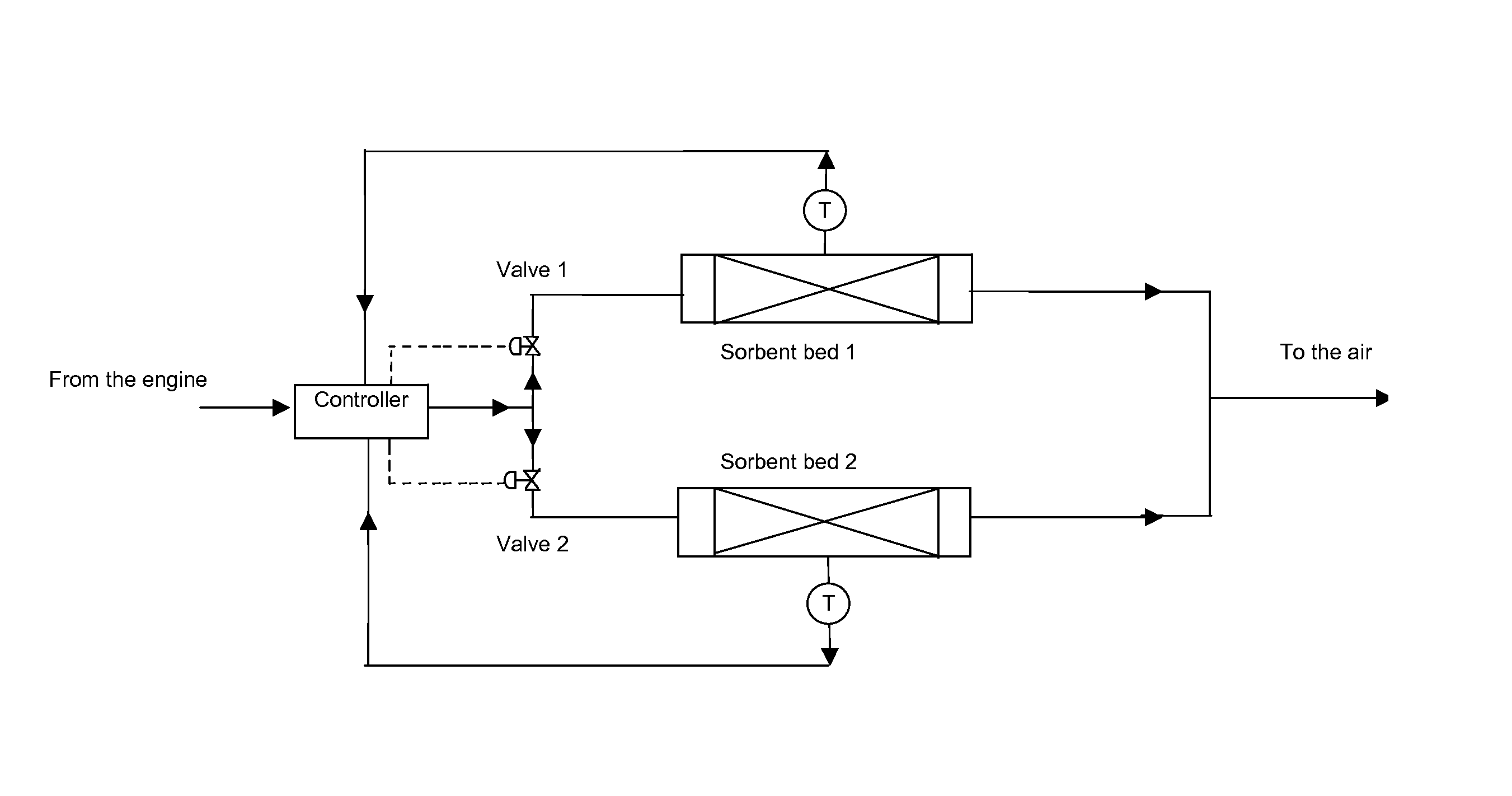
[0054]FIG. 4 shows a diagram with how a mixture of sorbent beds can be arranged in the exhaust system so that parallel desorption and adsorption cycles can be controlled by series of temperature sensors and valves to capture the CO2 on board, then the desorption can be controlled and the released CO2 sent to separate storage system. It is anticipated that such a capture system will be positioned after the catalytic elements of the exhaust system.
[0055]A combination of two types of sorbent could improve efficiency, one of them (like lithium zirconate) being placed in a chamber where the temperatures of the exhaust streams are higher and the second one (e.g. a h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com