Use of flagellins from the genus marinobacter as vaccination adjuvants
a flagellin and marinobacter technology, applied in the field of medicine, can solve the problems of inability to activate the immune system of bacteria flagellins, adverse secondary effects, and very susceptible birds and other animals to infection, and achieve the effects of low cross-reactivity, limited reactivity between flagellins, and limited cross-reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Obtainment and Purification of the Recombinant Flagellin Sequences
[0079]In this invention, we study the functionality and the adjuvant-vaccinal capacity of Marinobacter algicola flagellins F and FR (DG893T strain). In order to compare the activity and the function of the Marinobacter flagellins, we have also obtained and used Salmonella typhimurium flagellin STF.
[0080]The F and FR genes of the Marinobacter algicola flagellins were chemically synthesised (MrGene, Germany) from the primary DNA sequences of both flagellins, SEQ ID NO: 19 and SEQ ID NO: 21, respectively. Subsequently, a sequence that encodes a histidine tail was added to said sequences (in order to facilitate the purification of the recombinant protein obtained) and a sequence that encodes for the KDEL peptide was also added (it prevents the degradation of the recombinant protein obtained). On the other hand, starting from the Salmonella typhimurium DNA (GenBank: AY649721), the flagellin gene was obtained by polymerase ...
example 2
In Vitro Study of the Activity of Marinobacter Flagellins F and FR
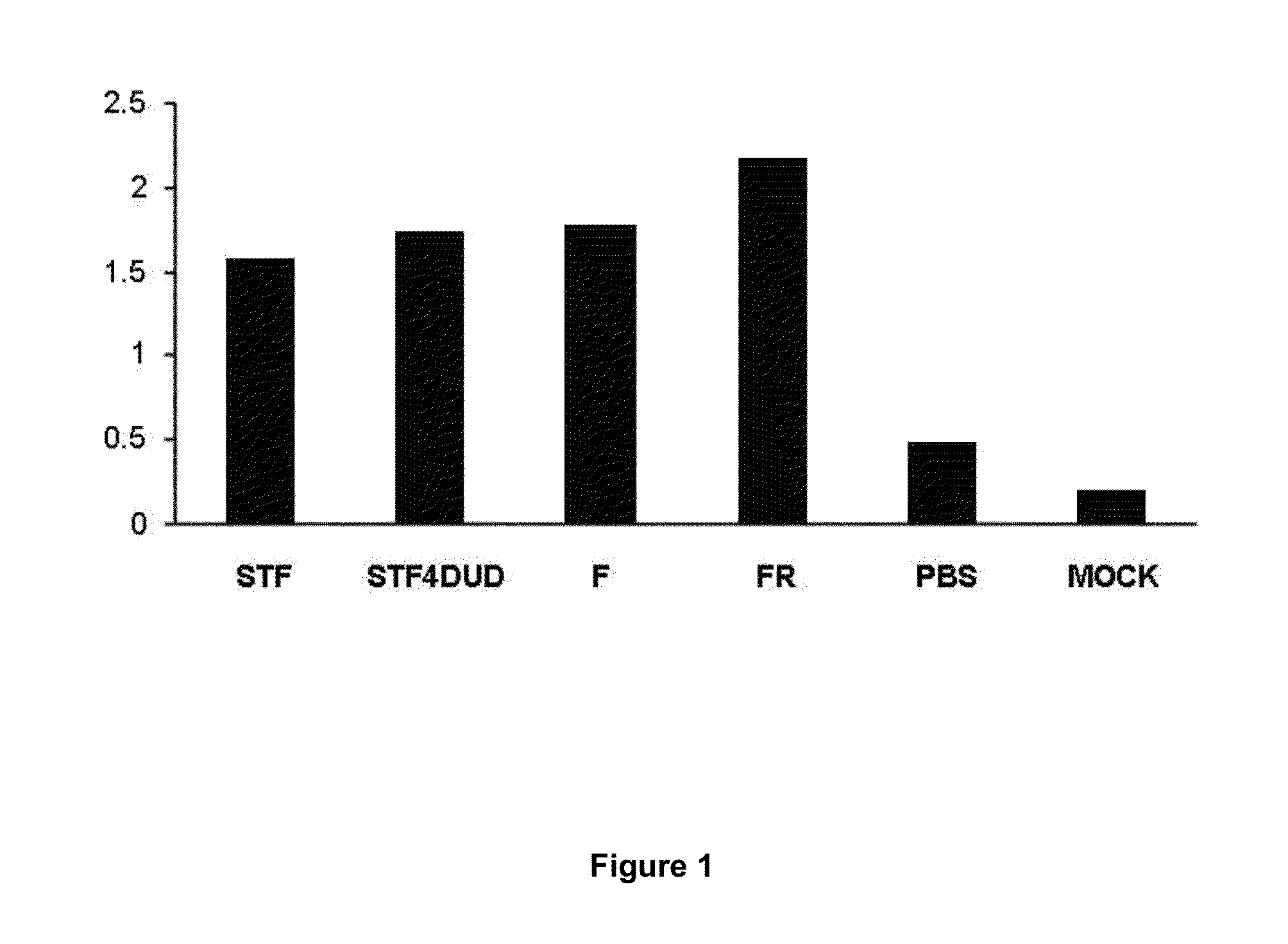
[0085]In order to analyse the in vitro activity of the recombinant Marinobacter flagellins F and FR obtained using the commercial “Bac-to-Bac® Baculovirus system” method (Invitrogen, USA), human CACO-2 colon carcinoma cells were used which constitutively express the TLR5 receptor. When TLR5 is activated in these cells, it induces the activation of MyD88 in order to finally secrete IL-8 to the extracellular medium [8-10].
[0086]CACO-2 cells (0.5 ml) were cultured in a 24-well plate (Nunc, Denmark) with an 80% confluence and a concentration of 5×105 cells / well. Subsequently, the recombinant proteins from flagellin STF (Salmonella typhimurium), used as a positive control, F and FR (Marinobacter algicola) at a final concentration of 1 μg / ml, were added to the culture medium, and incubated for 18 hours at 37° C. in a 5% CO2 atmosphere. Samples whereto nothing was added (MOCK) and others whereto PBS was added were used as ne...
example 3
“In Vivo” Study of the Vaccinal Capacity and the Immunogenicity of Marinobacter Fusion Flagellins F and FR Bound to 4 Tandem Copies of the DUD Epitope
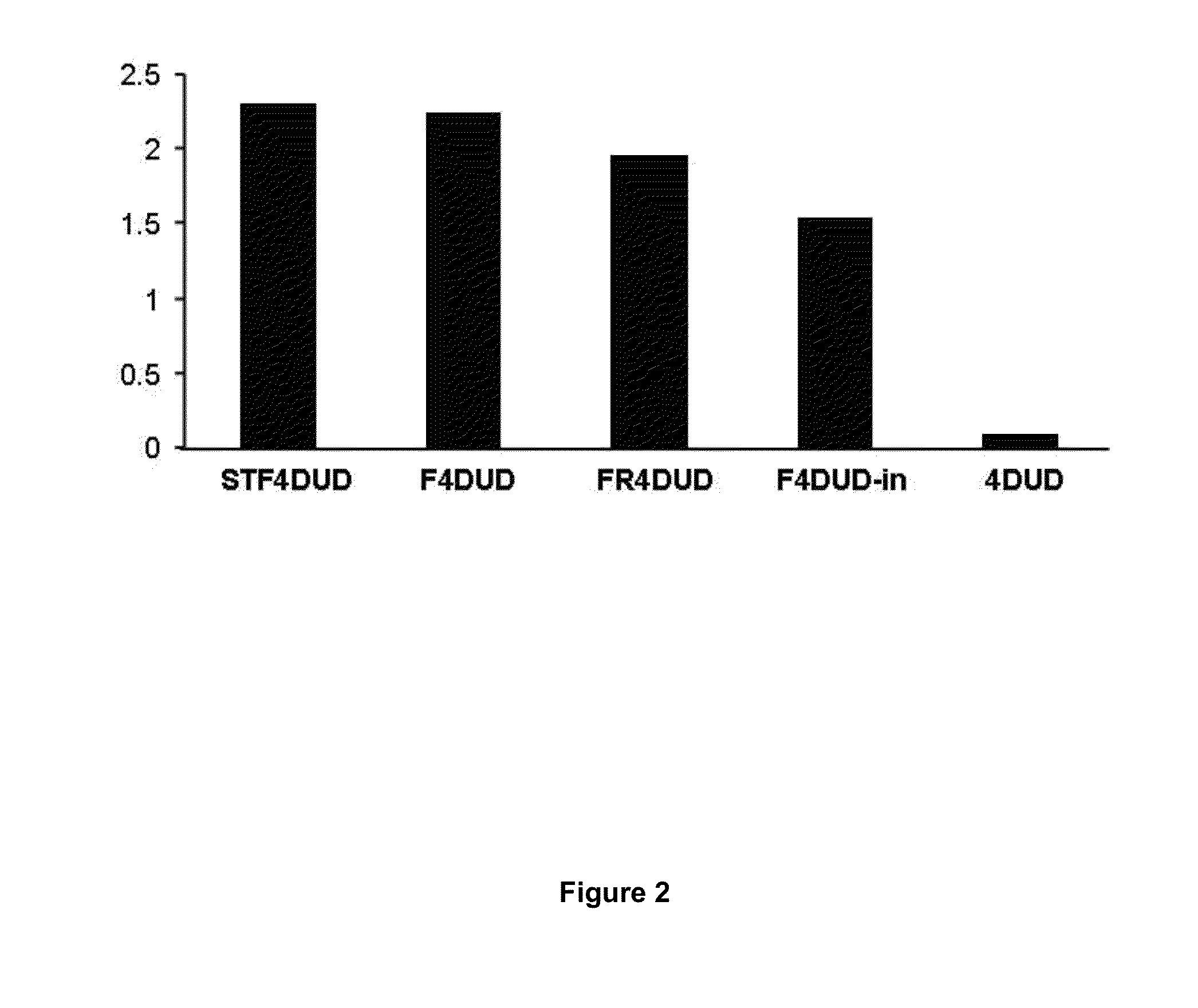
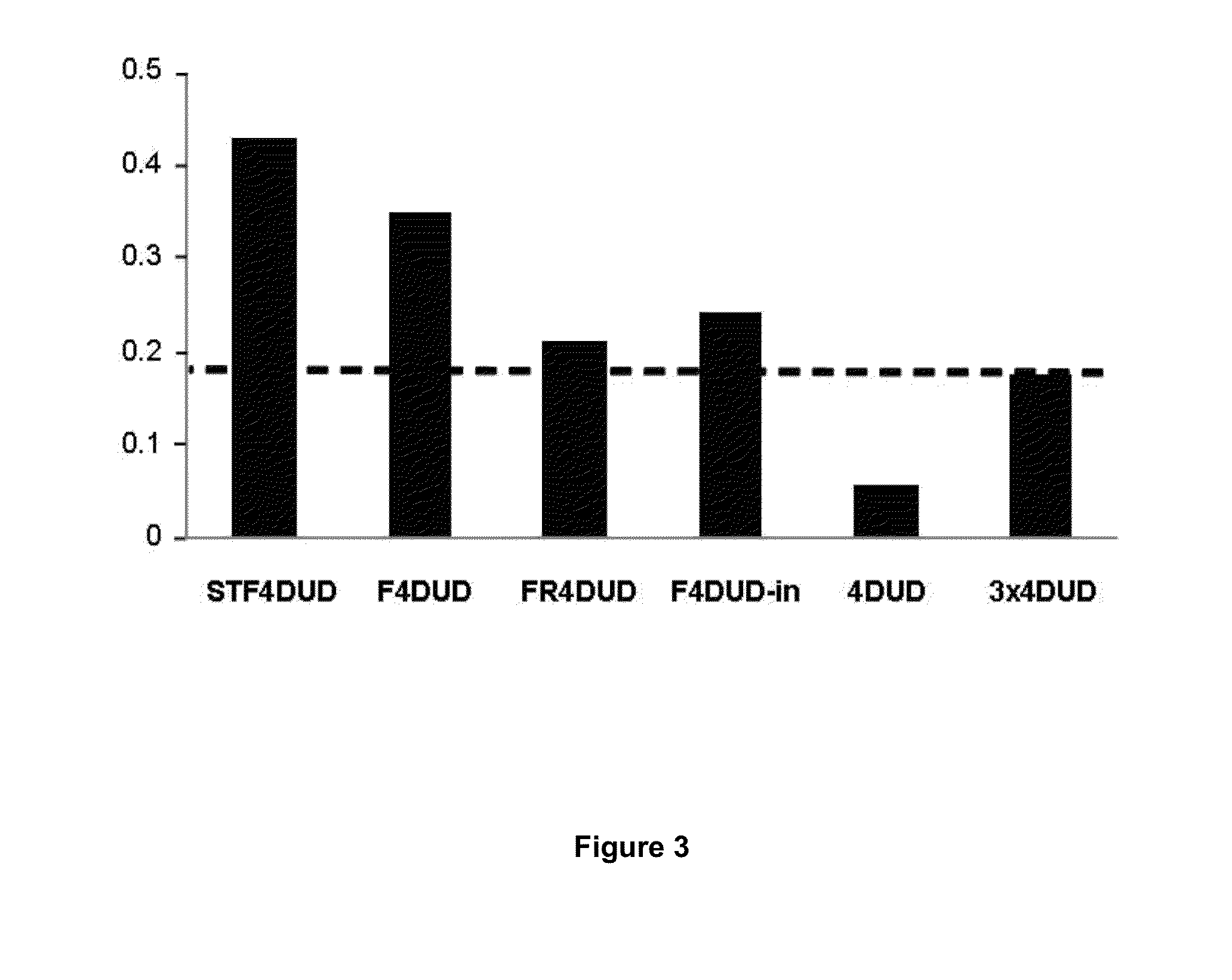
[0088]In order to analyse in vivo the vaccinal capacity and the immunogenicity of the Marinobacter flagellins F and FR obtained using the commercial “Bac-to-Bac® Baculovirus system” method (Invitrogen, USA), groups of 5 mice were immunised, by subcutaneous route (sc), with 30 μg of purified fusion flagellins STF4DUD (Salmonella typhimurium), F4DUD and FR4DUD (Marinobacter algicola). A group of 5 mice were also immunised by intranasal route (in) with the F4DUD protein. As a control group, 5 mice were inoculated with the DUD tetrapeptide (4DUD). 3 immunisations without adjuvants were applied on days 0, 15 and 30 of the assay, and blood samples were obtained from all the animals prior to each immunisation and 15 days following the last immunisation, although the serological studies of the presence of antibodies were performed with the ser...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com