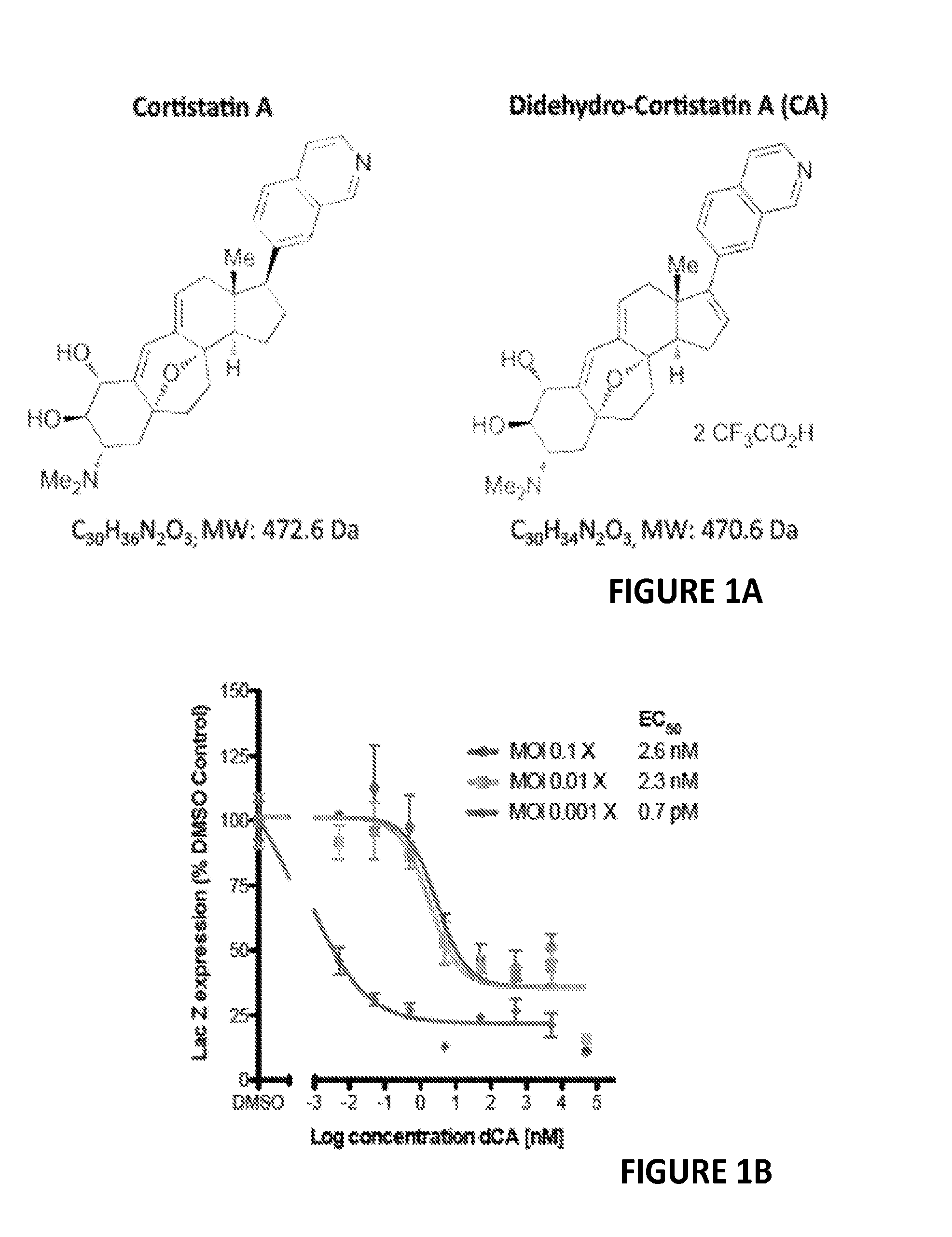
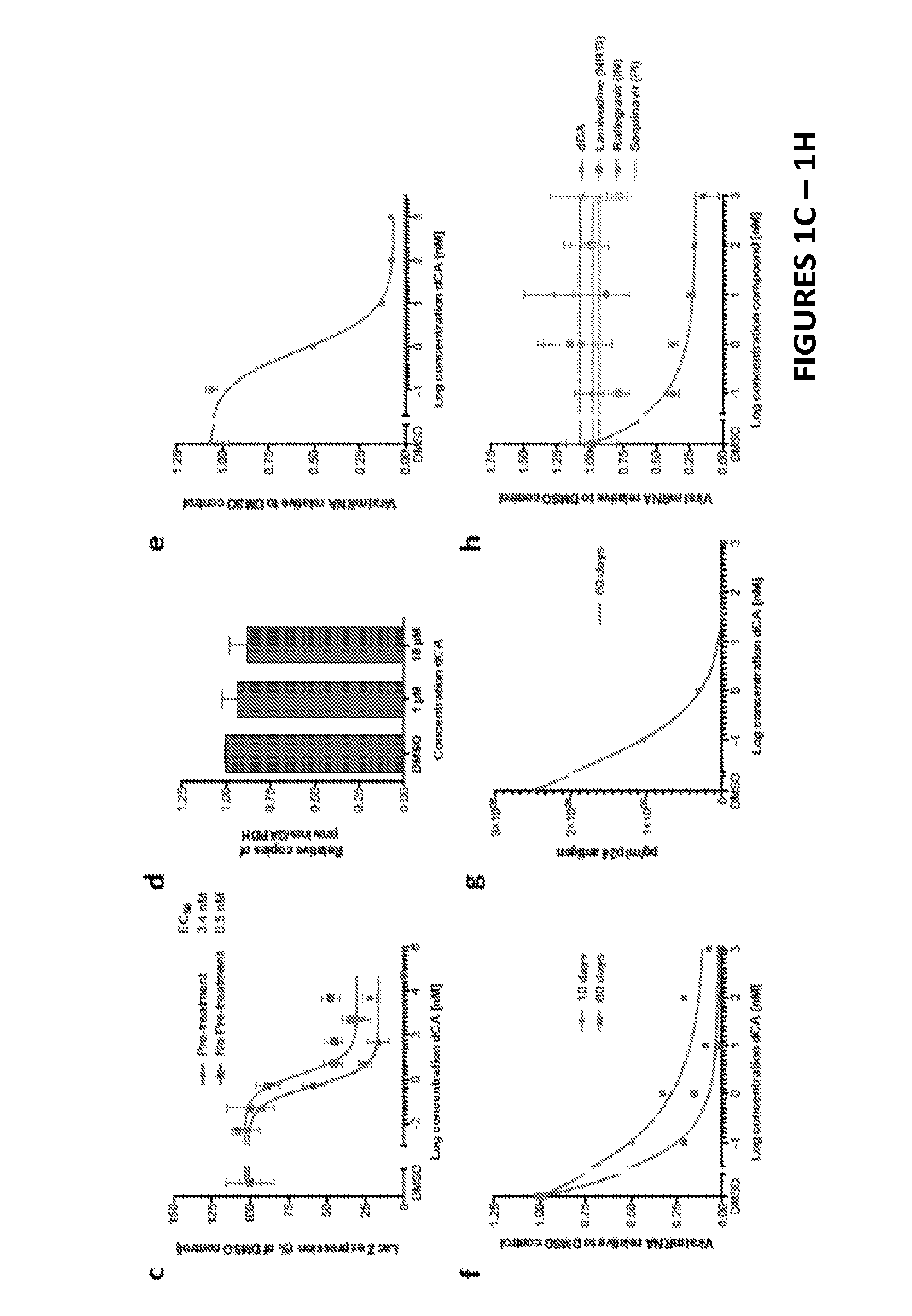
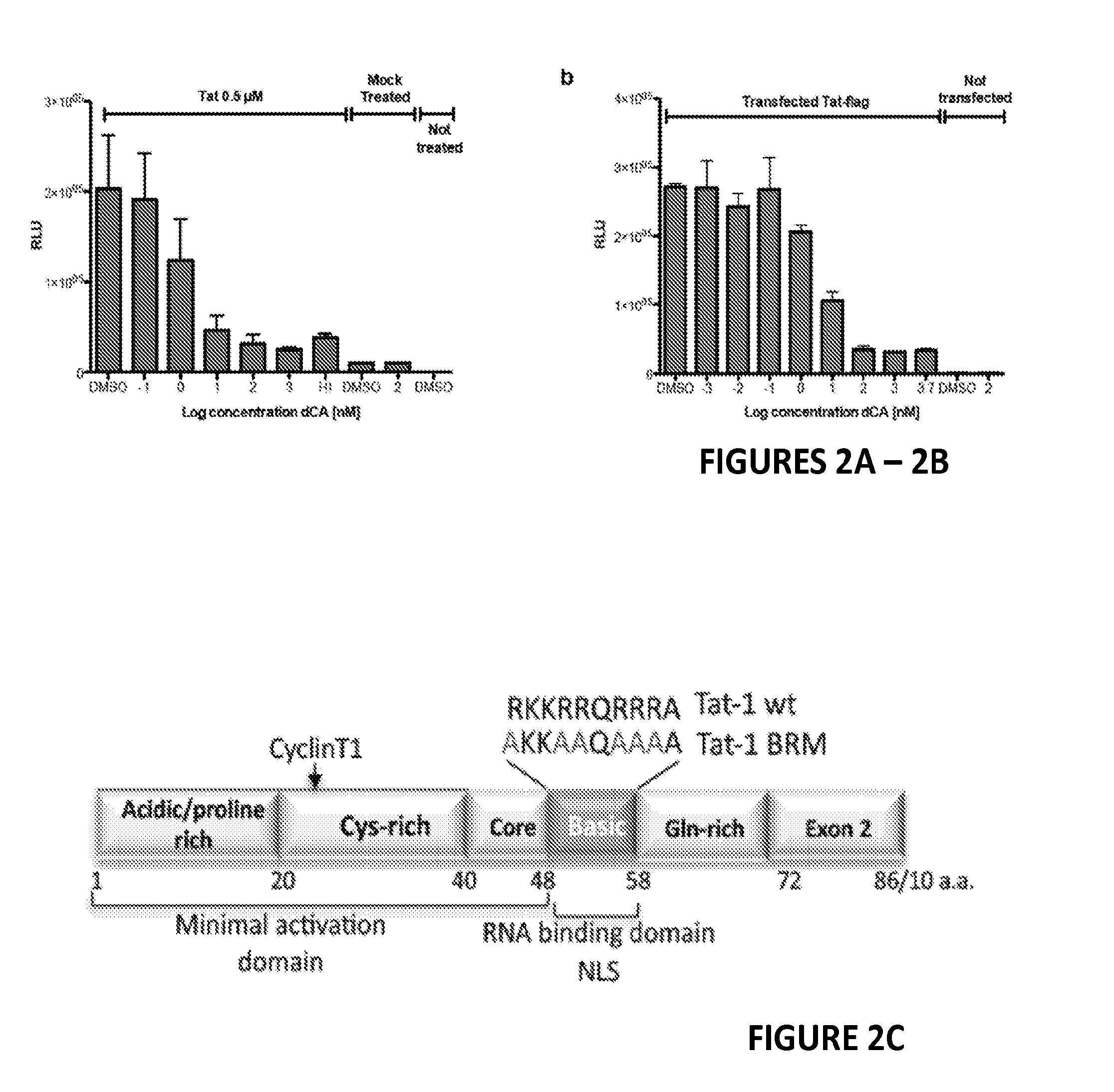
Inhibitors of Retroviral Replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Potent Suppression of HIV Viral Replication 1 by a Novel Inhibitor of Tat
[0188]Although treatment with antiretroviral drugs (ARVs) has extended the quality and expectancy of life for people infected with HIV, it has been unsuccessful in curing HIV infection. ARVs fall into the following major classes: fusion inhibitors (FIs), nucleoside reverse transcriptase inhibitors (NRTIs), non nucleoside reverse transcriptase inhibitors (NNRTIs), nucleotide reverse transcriptase inhibitors (NtRTIs), protease inhibitors (PIs) and integrase inhibitors (INIs). Highly active antiretroviral therapy (HAART) is based on triple or quadruple combinations of ARVs, however while reducing HIV to very low levels, this treatment fails to eliminate the infection completely. Ultrasensitive assays revealed that HIV persists in latently and productively infected CD4+ T cells in the peripheral blood of individuals receiving HAART who have maintained undetectable plasma viremia for prolonged periods of time. Resi...
example 2
Identification of CA-Associated Cellular Proteins
[0241]To identify cellular proteins associated with CA, a biotinylated form of the compound (Bio-CA) was used. This derivative did not compromise the viability of the cells, and it showed a 10 fold higher EC50 than CA, nevertheless at higher concentration it showed similar efficacy to CA. The Bio-CA was used to cover streptavidin-coated magnetic beads to pull down interacting proteins from either uninfected or chronically infected lysates of cells grown in the presence or absence of CA for 48 hours (FIG. 14D). The identity of the proteins pulled down under the different conditions was determined by HPLC MS / MS analysis. The proteins were identified with a greater than 95% confidence using a proprietary Scripps proteomics software package for protein identification, statistical analysis, and mass / peptide sequence correlation. The HIV proteome was added to the proteomics software search database before analysis. Many proteins were ident...
example 3
Modulation of Neurotoxicity
[0244]One of the more notable early events in HIV infection is how rapidly the virus is detected in the central nervous system (CNS). This may result in a variety of clinical abnormalities, including HIV-associated encephalitis (HIVE) and dementia (HAD), which occur in approximately 10-15% of patients chronically infected in the United States (A. V. Albright, et al., J. Neurovirol. 9 (2) (2003) 222-227; J. C. McArthur, et al., Lancet Neurol. 4 (9) (2005) 543-555). Despite the initial drop in the incidence of cognitive impairment as a result of highly active antiretroviral therapy (HAART), CNS disease is again increasing as HIV-infected individuals are living longer.
[0245]In the brain, macrophages / microglia are the primary cells infected by HIV, and these cells can support viral replication. A small percentage of astrocytes can also be infected, but HIV entry (B. Schweighardt, et al., AIDS Res. Hum. Retroviruses, 17 (12) (2001) 1133-1142) and replication (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com