Soft tissue repair allografts and methods for preparing same
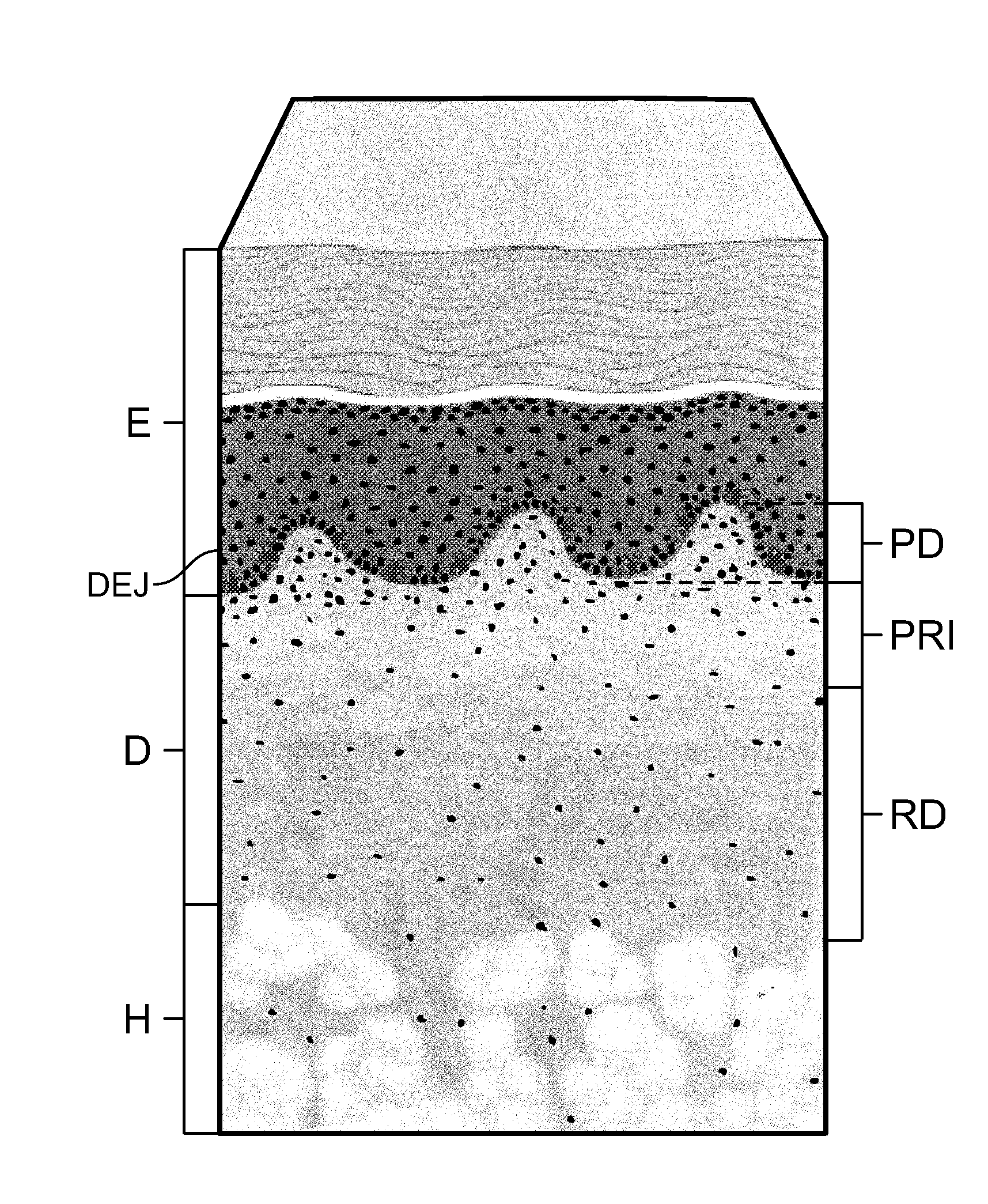
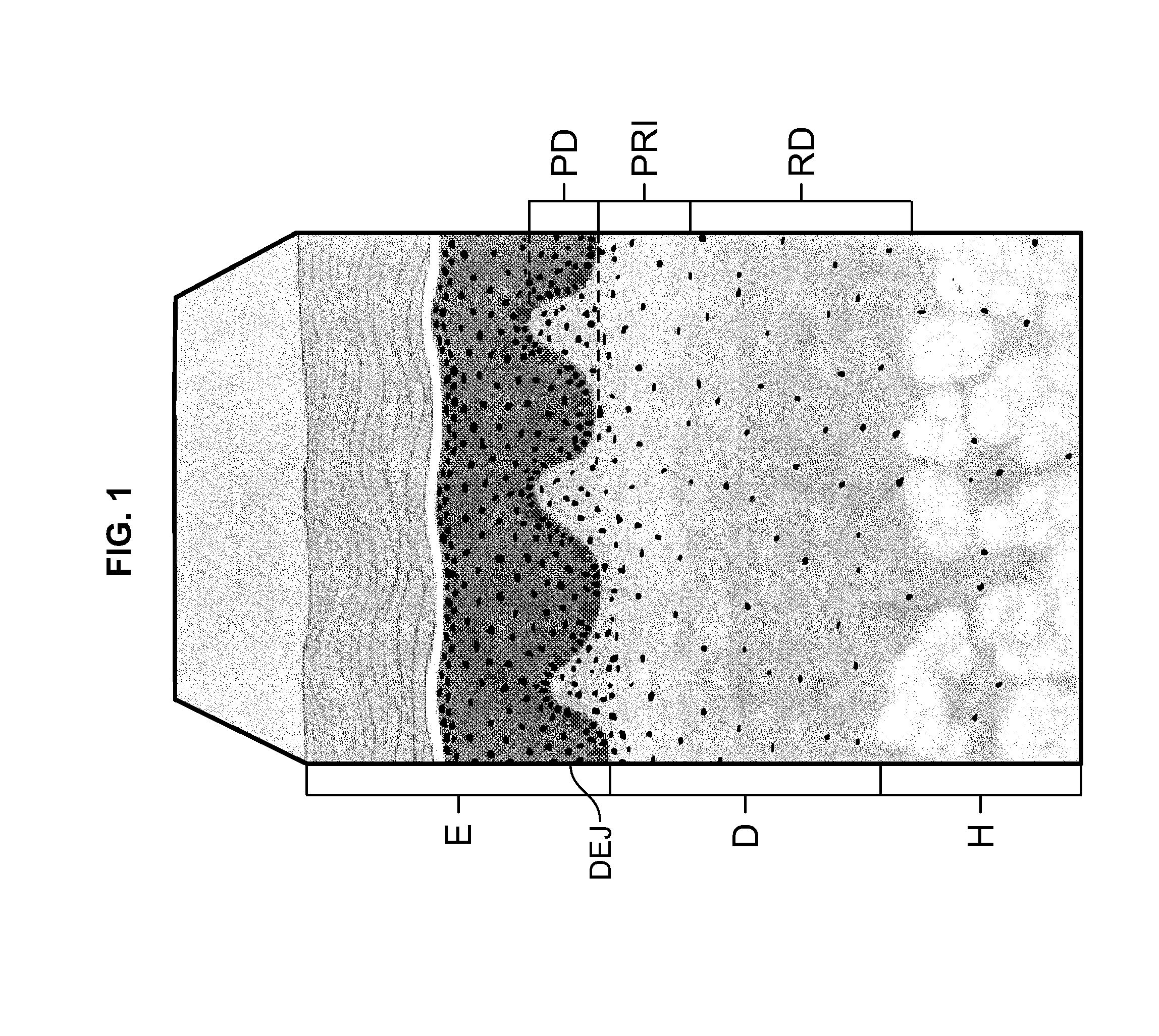
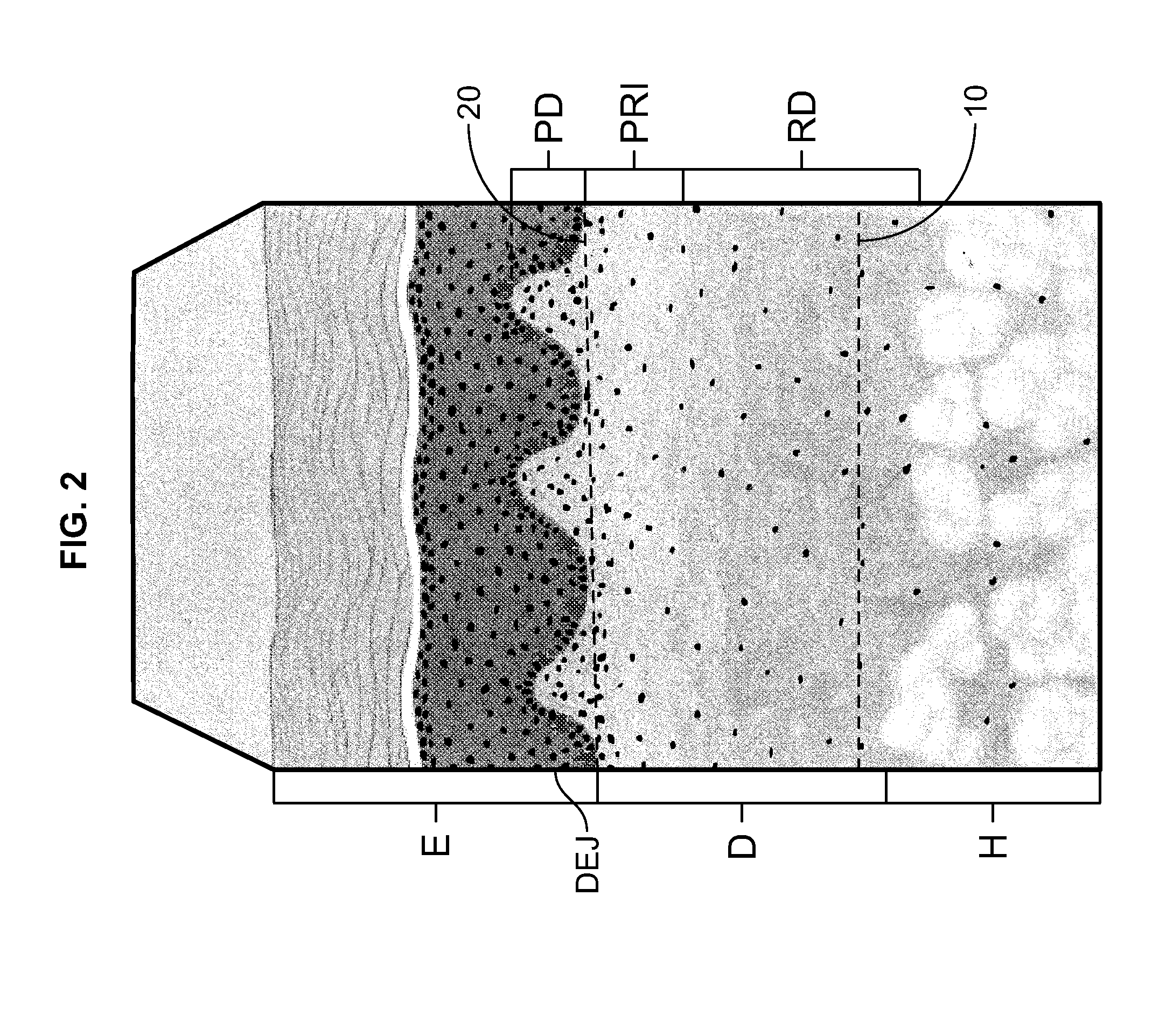
a soft tissue repair and allograft technology, applied in the field of allografts, can solve the problems of uniform microstructure of the papillary dermis pd, and achieve the effect of uniform density and porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Fibroblast Attachment to the ACDMs
Materials and Methods
[0037]7 mm punches of each tissue sample (i.e., each ACDM) were prepared and seeded with 1×105 BJ neonatal human foreskin fibroblasts (ATCC, Manassa, Va.) on both sides in Eagles Minimum Essential Medium +10% fetal bovine serum. After 30 minutes, the tissue sections were washed to remove any non-adherent cells and incubated at 37° C. for 1 hour in complete growth medium. Attached cells were quantified using CyQuant Cell Proliferation Assay (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Non-adherent seeded controls were measured for all samples. The test was replicated with each sample set. Values for cell fluorescence were reported. Tissue from multiple donor lots were collected, processed as described and tested. In addition, five lots of AlloDerm® RTU thick tissue were obtained and tested as commercial controls.
Results
[0038]
TABLE 1In vitro fibroblast attachmentNo. ofSamplesCells*Grouping*...
example 2
Tensile Properties of the ACDMs
Materials and Methods
[0046]Tissue samples (i.e., for each ACDM) were tested on an MTS 858 Mini Bionix System. Sample thickness was first measured with a laser micrometer (Z Mike, Benchmike 4050S). Samples in dogbone configuration (1 cm×7 cm; ASTM 638) were positioned in pneumatic action grips set at 29 psi pressure at a gage length of 26 mm. Tissue was pulled to break at a strain rate of 50.6 mm / min. Ultimate tensile strength, elongation-at-break and elastic modulus were recorded. Statistical analysis included both tests of the means and the estimates of variability for tensile strength, elongation-at-break, and modulus.
Results
[0047]As a result of the more open structure and greater porosity of the Disclosed ACDM, as contrasted with the FlexHD Structural ACDM, the Disclosed ACDM has reduced tensile strength as compared to the FlexHD Structural ACDM; 10.97 vs. 15.36 MPa.
[0048]As can be seen from the data in Table 2 and the graph illustrated in FIG. 7, t...
example 3
Surface Characterization of the ACDMs by Scanning Electron Microscopy (SEM)
Materials and Methods
[0055]Tissue samples (i.e., for the Disclosed ACDM and the FlexHD Structural ACDM) were lyophilized and coated with a 10 nm layer of gold. Images were taken using a Field Emission Zeiss Scanning Microscope (Carl Zeiss, Inc., Thornwood, N.Y.) with a working distance of 5-10 mm and voltage range of 30-200 kV. All images were taken at the Department of Ceramics and Material Science at Rutgers University, New Brunswick, N.J.
Results
[0056]Scanning electron micrographs of the epidermal side and the dermal side of both the FlexHD Structural ACDM and the Disclosed ACDM are presented in FIGS. 8a and 8b, respectively. Representative images were taken at 250× for all samples. For both ACDMs, the micrographs of the epidermal side of the ACDMs are shown on the left, and the micrographs of the dermal side are shown on the right.
Discussion
[0057]The deeper cut method of the present invention that was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com