Novel Target for Diagnosis and Treatment of Diabetes and Cardiovascular Diseases
a cardiovascular disease and new target technology, applied in the field of diabetes and cardiovascular disease diagnosis and treatment, can solve the problems of chronic hyperglycemic diabetic state, endocrine effects on distant tissues, and significant decrease in beta cell function and mass, and achieve the effects of improving diabetes, diagnosing and prognosing insulin secretion deficiency and/or beta cell dysfunction, and stimulating insulin secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Procedures
Animals and Treatments
[0062]7 week old male C57BL / 6N and CX3CR1 knockout mice were purchased from Taconic (USA). GTT and ITT results from the CX3CR1 KO mice were confirmed in CX3CR1gfp / gfP knockin mice obtained from Jackson Laboratory (USA). All the mice were housed in colony cages in 12 h light / 12 h dark cycles. For HFD study, 8 week old mice were subjected to NCD or 60% HFD (Research Diets, Inc; USA). For oral glucose tolerance test, the mice were fasted for 6 h and basal blood samples were taken, followed by oral gavage of 2 g / kg bolus glucose. Blood samples were drawn at 10, 20, 30, 45, 60, 90 and 120 min after oral gavage. For intravenial glucose tolerance test, the mice were surgerized for cathether implantation to jugular vein as described previously (Lee et al., 2011). After 3 days of recovery, the mice were fasted for 5 h, and then transferred to restrainers. After 1.5 h of stabilization, blood samples were drawn from tail for basal glucose and insuli...
example 2
CX3CR1-Deficient Mice Exhibit Impaired Glucose Tolerance with Reduced Insulin Secretion
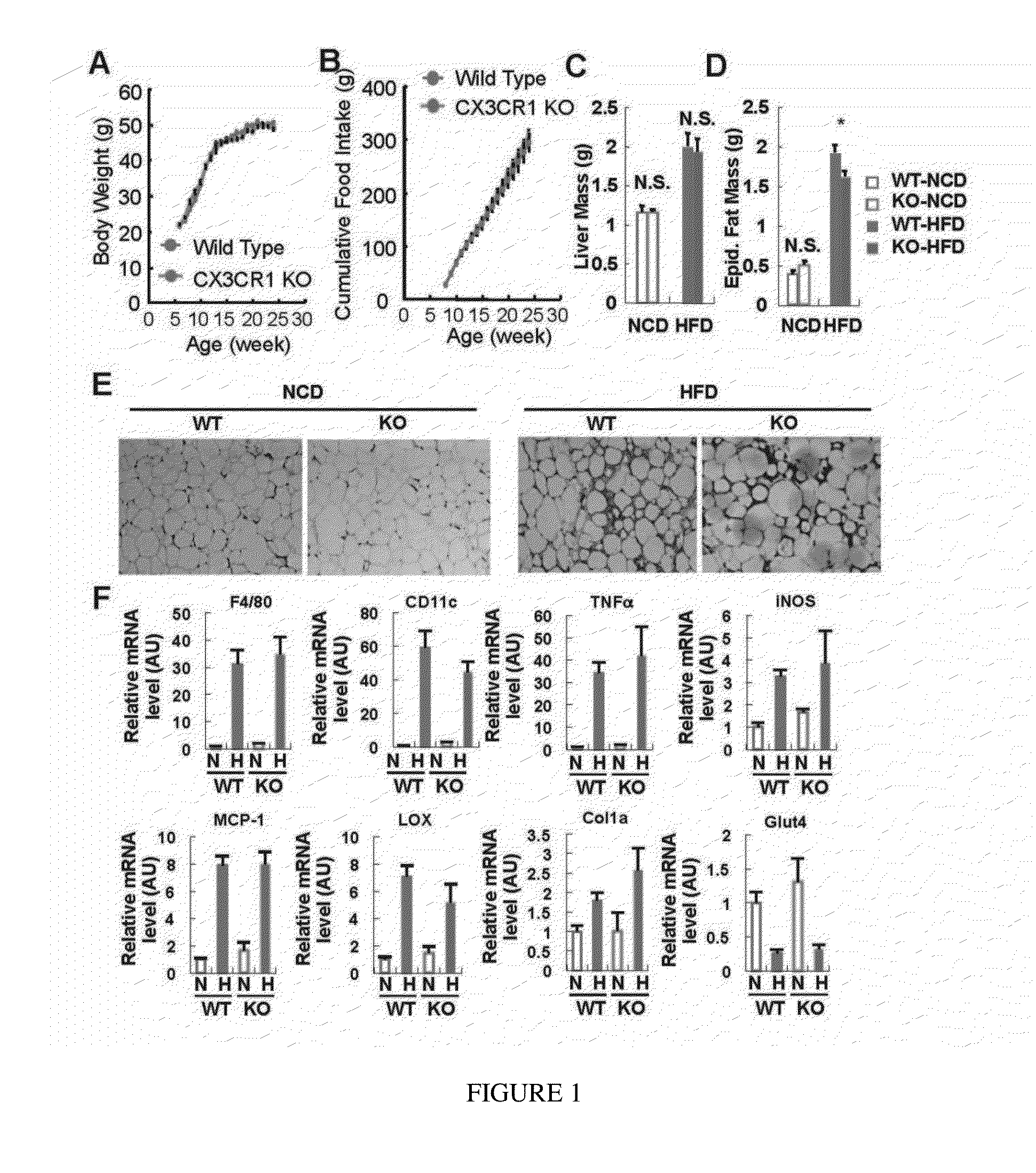
[0072]Obesity causes inflammation and insulin resistance and the FKN / CX3CR1 system plays a role in monocyte attachment and immune cell migration (Combadiere et al., 2003; Hotamisligil et al., 1995; Lee et al., 2010; Lee et al., 2011; Lesnik et al., 2003; Olefsky and Glass, 2010; Tacke et al., 2007; Teupser et al., 2004). To address the potential effect of this receptor on obesity-induced inflammation, lean and obese CX3CR1 KO mice were studied. The KO mice exhibited normal food intake, body weight gain, and liver mass either on chow or high fat diet (HFD) (FIGS. 1A-1C). Adipose tissue mass was the same between KO and WT mice on chow diets, but was slightly lower in the KO mice on HFD (FIG. 1D).
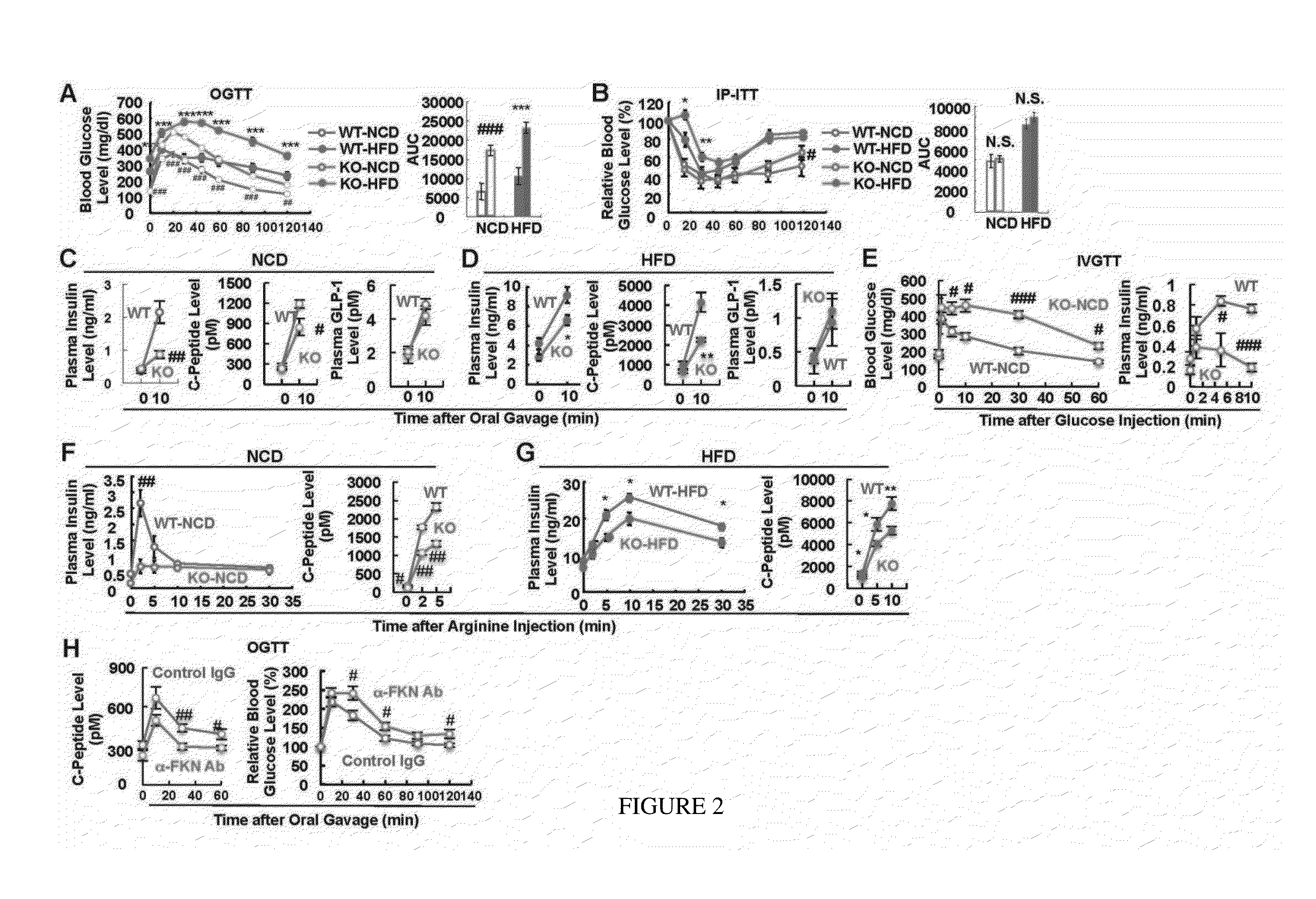
[0073]Interestingly, it was found no evidence that FKN or CX3CR1 play a role in macrophage accumulation in adipose tissue or liver or in inflammation-induced insulin resistance. For example, macrophage infil...
example 3
CX3CR1 KO Islets Display Reduced Insulin Secretion with Decreased Expression of Genes Associated with Beta Cell Function
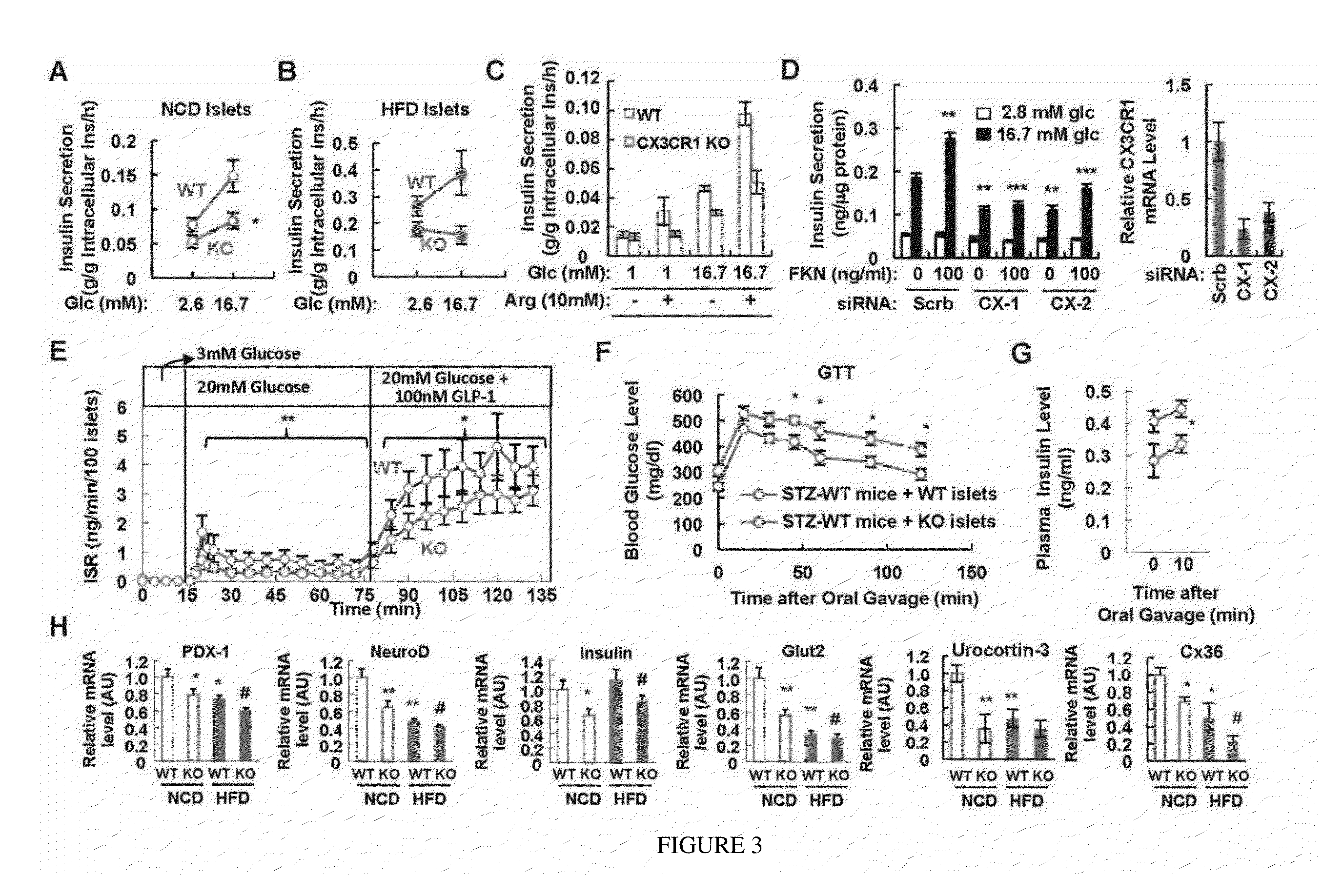
[0076]To directly test beta cell function, and to determine whether the in vivo insulin secretory defects are primary or secondary, in vitro systems on isolated islets and Min6 cells were carried out. First, to directly test the function of CX3CR1 in beta cells, insulin secretion was measured under static incubation conditions by isolated islets obtained from chow and HFD WT and CX3CR1 KO mice. As seen in FIG. 3A, the KO islets exhibited a marked decrease in GSIS which was more profound in islets obtained from HFD KO mice (FIG. 3B). Moreover, CX3CR1 KO islets exhibited reduced insulin secretion in response to arginine (FIG. 3C), consistent with the in vivo results seen in FIG. 2F. To demonstrate the cell autonomous effects of CX3CR1 on GSIS in another way, and to show that they are independent of in vivo developmental issues, RNAi interference was used to deplete C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility (mass) | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com