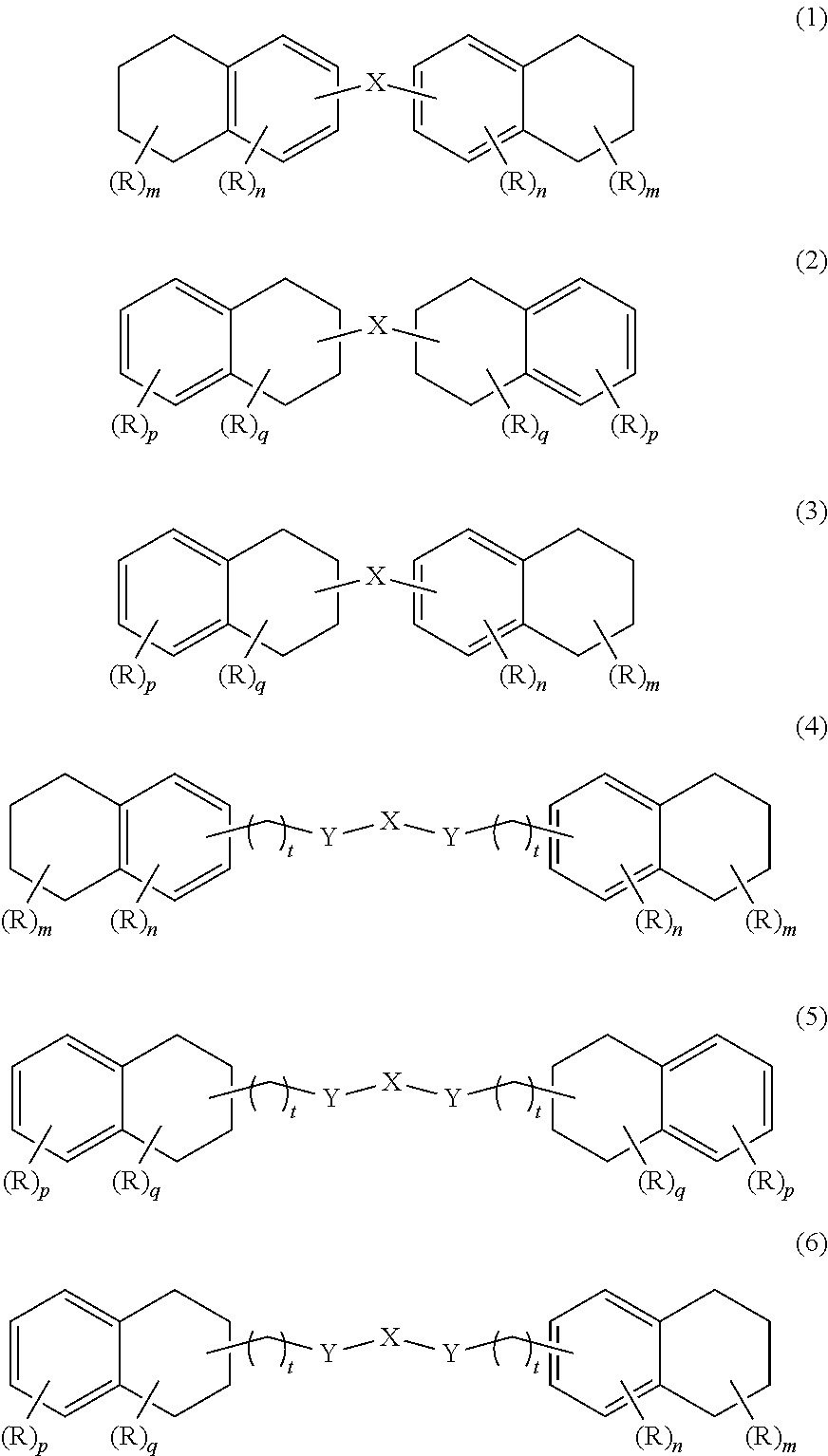
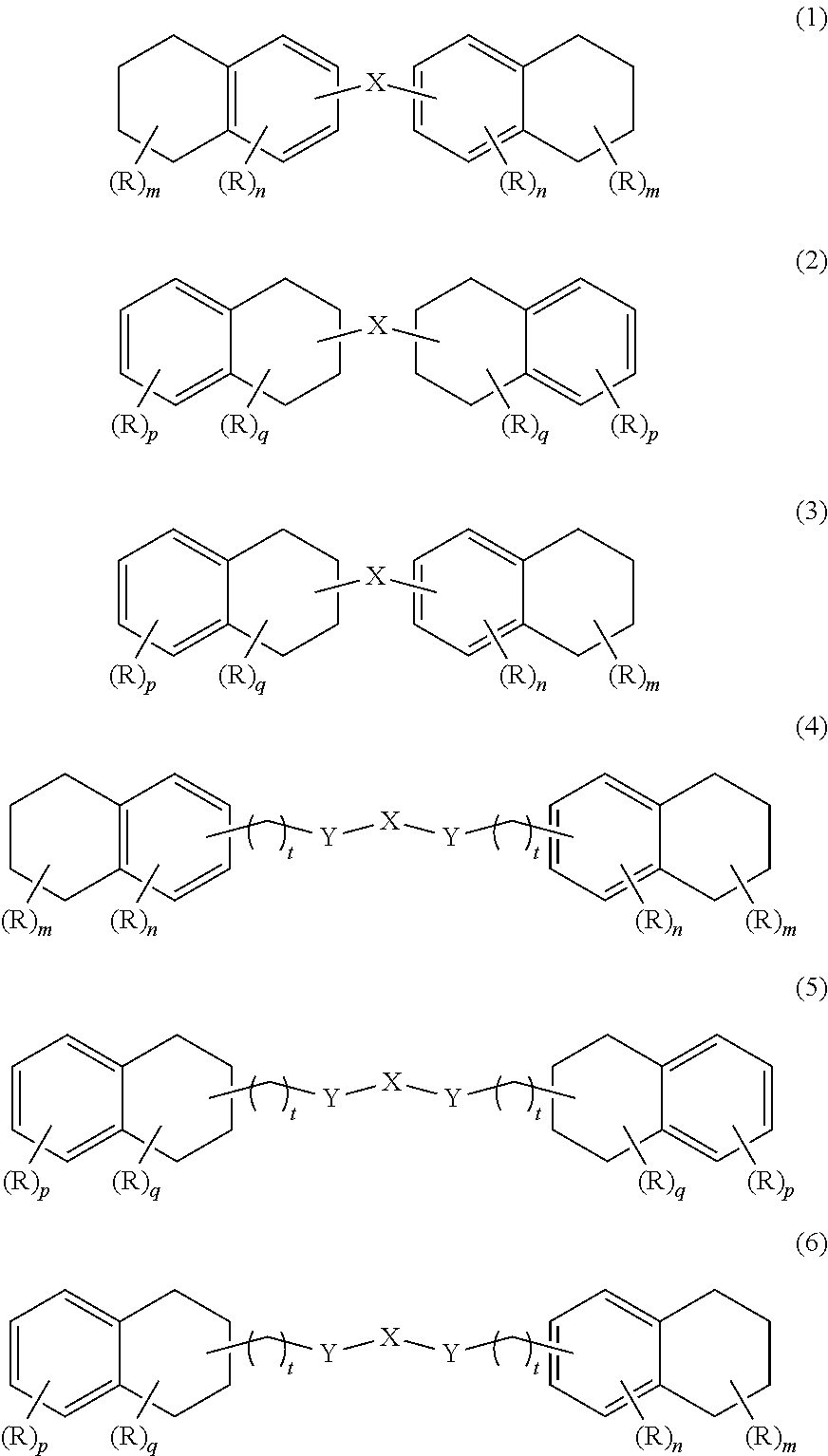
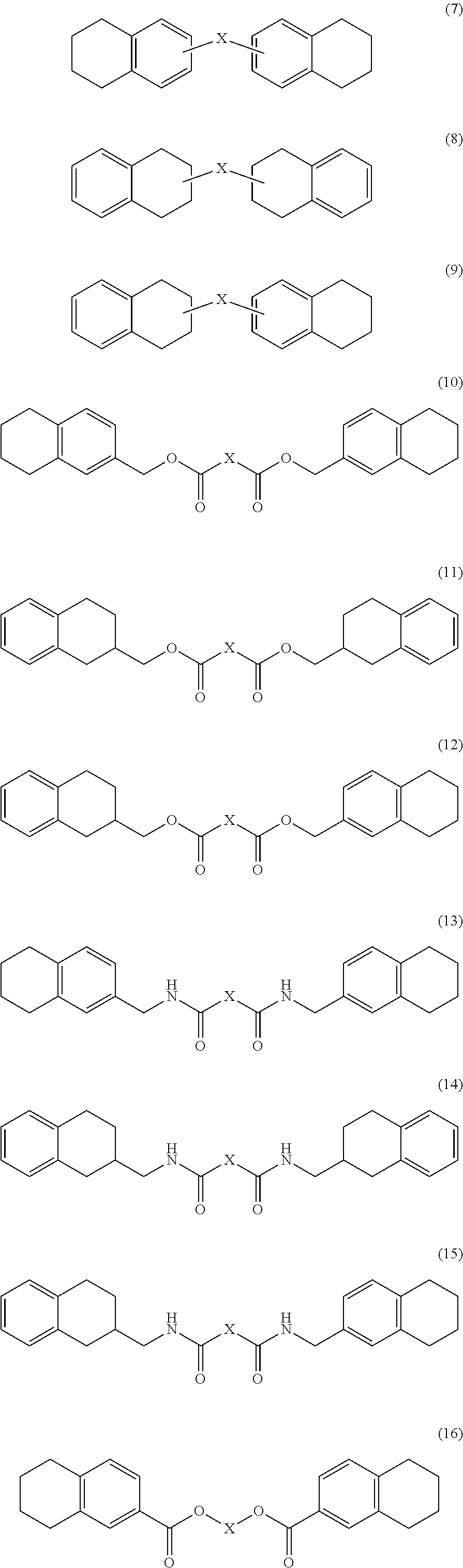
Oxygen absorber composition and oxygen absorber package
a technology of oxygen absorbing agent and oxygen absorbing agent, which is applied in the direction of metal/metal-oxide/metal-hydroxide catalyst, metal/metal-oxide/metal-hydroxide catalyst, etc., can solve the problem of difficult foreign matter inspection by using metal detectors, limited oxygen absorbing agent objects of iron-based oxygen absorbing agents, and inability to heat with microwave ovens. problems, to achieve the effect of excellent oxygen absorption performance, excellent oxygen absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Diester Compound A Having Tetralin Rings
[0091]In a 300-mL reactor equipped with a thermometer, a partial condenser, a total condenser and a stirring device, 10.8 g (62 mmol) of dimethyl adipate, 30.0 g (185 mmol) of tetralinmethanol were placed, and the reaction mixture was increased in temperature to 130° C. To the reaction mixture, 58 mg (200 ppm in terms of Ti) of titanium tetrabutoxide monomer was added, and then reaction mixture was increased in temperature to 200° C. and was allowed to react while the produced methanol was being removed from the reaction system. After the production of methanol ceased, the reaction mixture was cooled to room temperature, the unreacted tetralinmethanol was removed under reduced pressure, and the diester compound A was obtained by recrystallization. By using a thermogravimetric / differential thermal analyzer (trade name “DTG-60,” manufactured by Shimadzu Corp.), the 3% weight loss temperature of the obtained compound was measured. The measurement...
synthesis example 2
Diester Compound B Having Tetralin Rings
[0092]The diester compound B was obtained by performing the same operations as in Synthesis Example 1 except that dimethyl sebacate was used in place of dimethyl adipate and the weight of dimethyl sebacate was set at 14.3 g (62 mmol). The structural formula and the 3% weight loss temperature of the obtained compound are shown in Table 1. As an example, the results of the NMR analysis are shown below. 1H-NMR (500 MHz CDCl3) δ 7.03-7.10 (6H m), 5.50 (4H s), 2.68-2.79 (8H m), 2.26-2.39 (4H m), 1.73-1.84 (8H m), 1.60-1.69 (4H m), 1.25-1.34 (8H m).
synthesis example 3
Diester Compound C Having Tetralin Rings
[0093]The diester compound C was obtained by performing the same operations as in Synthesis Example 1 except that dimethyl succinate was used in place of dimethyl adipate and the weight of dimethyl succinate was set at 9.1 g (62 mmol). The structural formula and the 3% weight loss temperature of the obtained compound are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight loss temperature | aaaaa | aaaaa |
| weight loss temperature | aaaaa | aaaaa |
| weight loss temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com