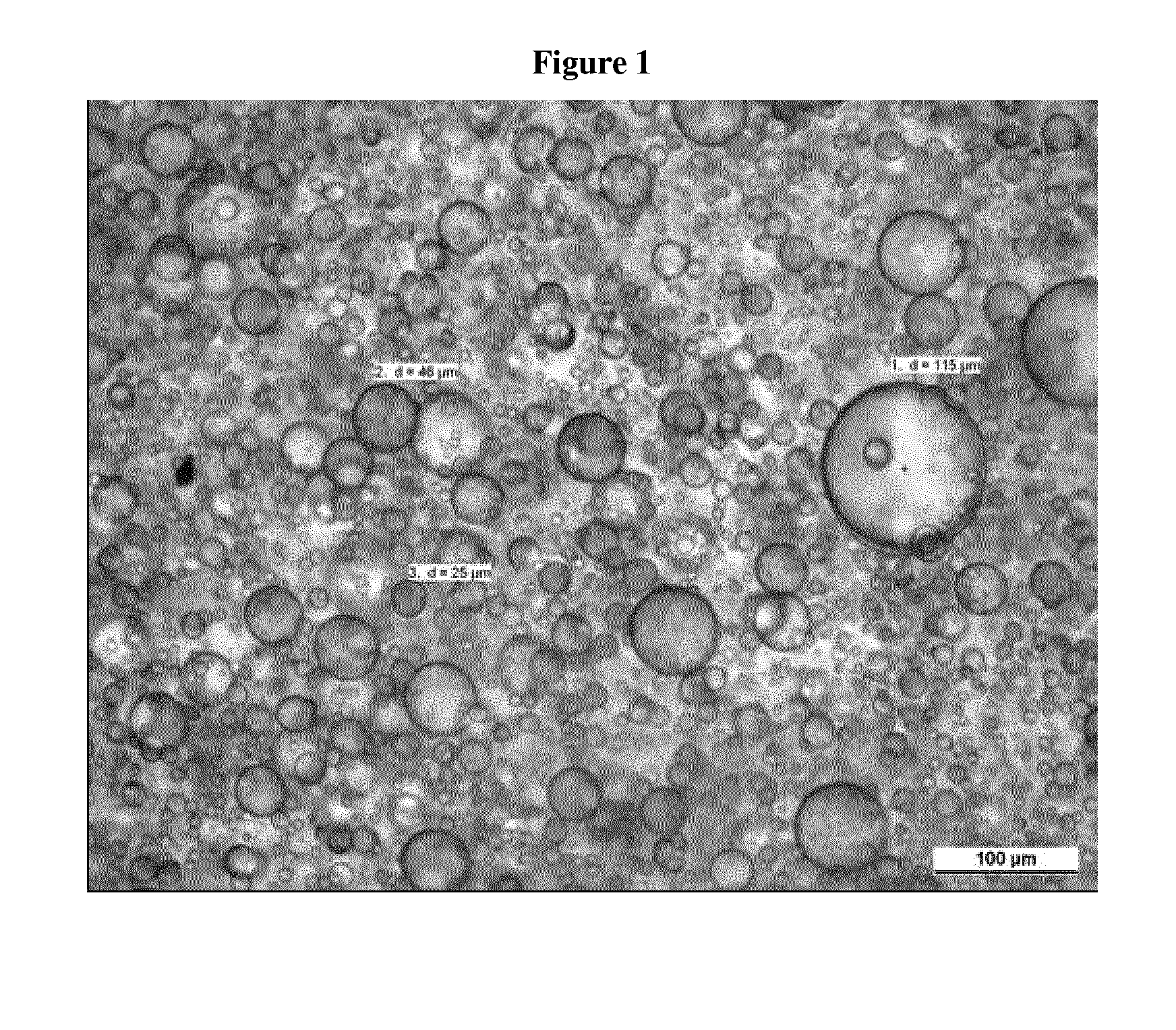
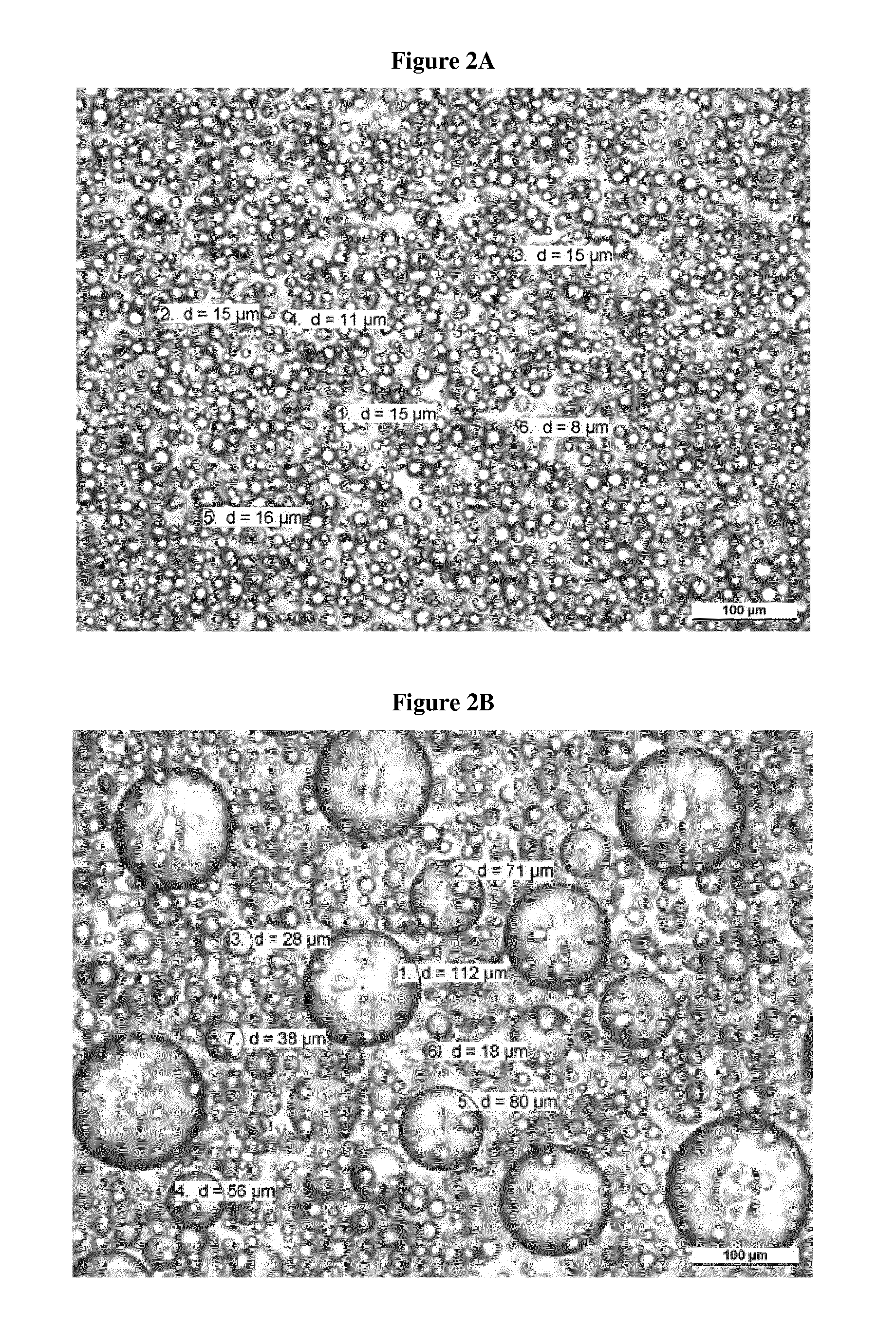
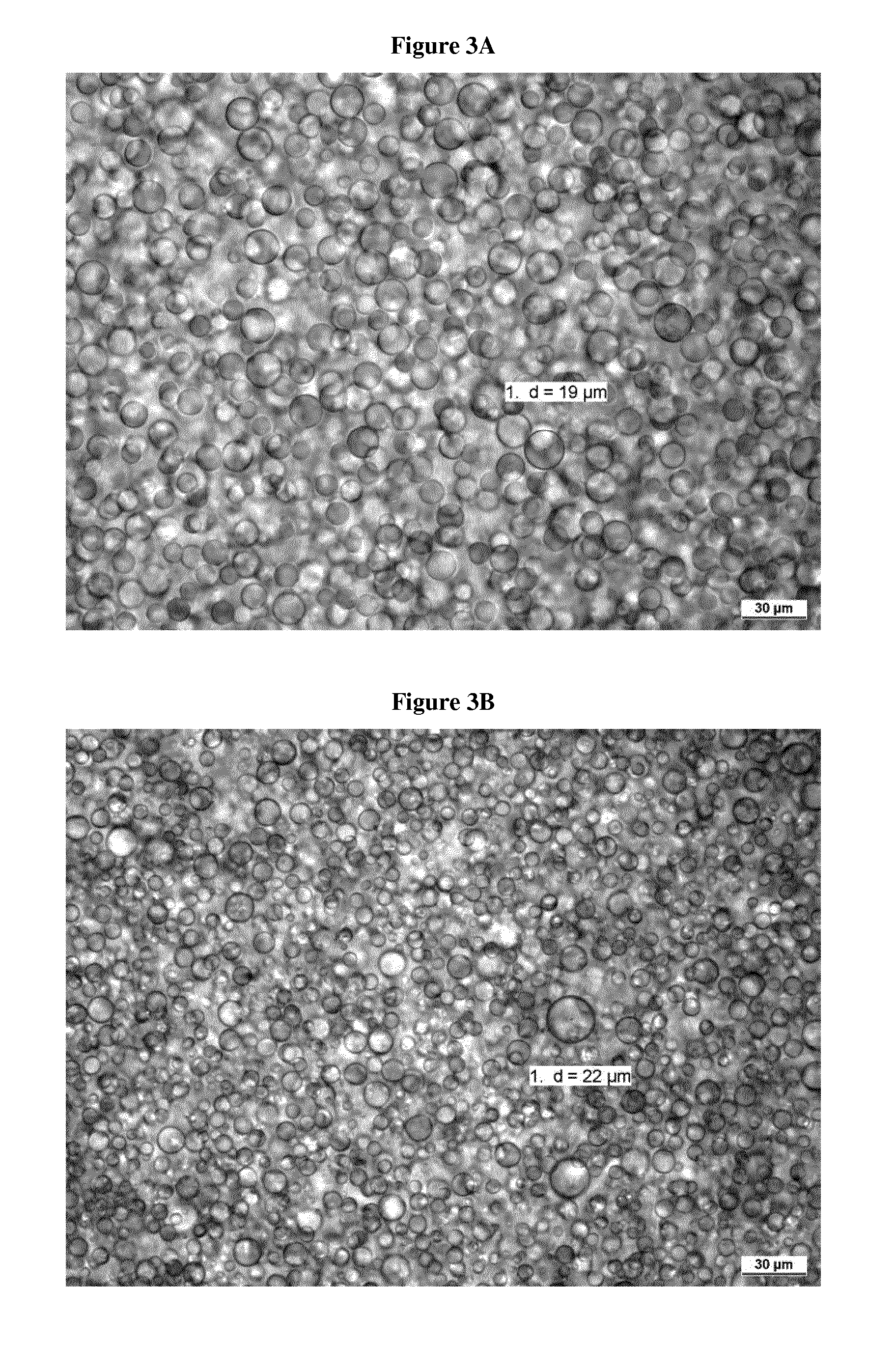
Transdermal delivery system
a technology of transdermal delivery and delivery system, which is applied in the direction of bandages, biocide, drug compositions, etc., can solve the problems of high variability in performance, system failure, undesired burst, etc., and achieve the effect of small area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3
[0285]The composition of the buprenorphine base-containing adhesive mixture is summarized in Table 3 below.
TABLE 3Ingredient (Trade Name)Amt / unit (g)Buprenorphine base7.44Levulinic acid5.24Ascorbyl palmitate0.14Polyvinylpyrrolidone (PVP)1.88Ethanol10.77Polysiloxane adhesive in n-heptane82.47Solids content of 73% by weight(BIO-PSA 7-4201 from Dow CorningHealthcare)n-heptane1.73Total109.67
[0286]In a suitable vessel, 37.86 g of polyvinylpyrrolidone and 113.57 g of ethanol were dissolved to form a 25% PVP pre-solution. The prescribed amount of the PVP pre-solution, Levulinic acid and Ascorbyl palmitate were suspended with stirring and afterwards the remaining part of Ethanol and the Buprenorphine was added to form a buprenorphine containing solution by stirring until a solution is formed. 82.50 g of a polysiloxane adhesive in the form of a solution in n-heptane having a solids content of 73% by weight and 1.74 g of heptane were added. The mixture was stirred to give 109.67 g of a bupren...
example 4
[0290]The composition of the buprenorphine base-containing adhesive mixture is summarized in Table 4 below.
TABLE 4Ingredient (Trade Name)Amt / unit (kg)Buprenorphine base1.368Levulinic acid0.958Polyvinylpyrrolidone (PVP)0.342Ascorbyl palmitate0.027Ethanol1.938Polysiloxane adhesive in n-heptane15.048Solids content of 73% by weight(BIO-PSA 7-4201 from Dow CorningHealthcare)n-heptane0.319Total20
[0291]In a 10 l vessel, 1.00 kg of polyvinylpyrrolidone and 3.00 g of ethanol were dissolved to form a 25% PVP pre-solution. In a homogenizing / mixing vessel: Becomix Lab mixer RW 30 Ex, 1.368 kg of PVP pre-solution, 0.958 kg levulinic acid, 0.027 kg of Ascorbyl palmitate and the main part of 0.912 kg of Ethanol were suspended by stirring. The prescribed amount of buprenorphine was weighed and added to the homogenizing / mixing vessel followed by rinsing the weighing container used for buprenorphine with the remaining part of Ethanol. The mixture was kept under stirring for at least 1 h until a bupre...
example 5
[0297]The composition of the buprenorphine base-containing adhesive mixture and the process of manufacture was as described for Example 4. After the mixing step forming a buprenorphine containing mixture, the buprenorphine base-containing adhesive mixture was additionally homogenized at a homogenizing speed of 2000 rpm-2500 rpm before coated on a polyethylene terephthalate film (e.g. Scotchpak from 3M).
[0298]In Example 5, films with two different coating weights of the matrix layer were prepared:
TABLE 5Coating weight of theExample 5matrix layer [g / m2]Example 5.1120Example 5.290
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com