Compositions and methods for delivery of immune cells to treat un-resectable or non-resected tumor cells and tumor relapse
a technology of immune cells and tumors, applied in the field of immune cells for treating unresectable or non-resected tumor cells and tumor relapse, can solve the problems of cancer relapse after surgery, cancer relapse remains cancer relapse is often a major clinical problem, so as to achieve the effect of effective treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation and Ex Vivo Expansion of 4T1 Breast Tumor-Reactive Mouse T-Cells
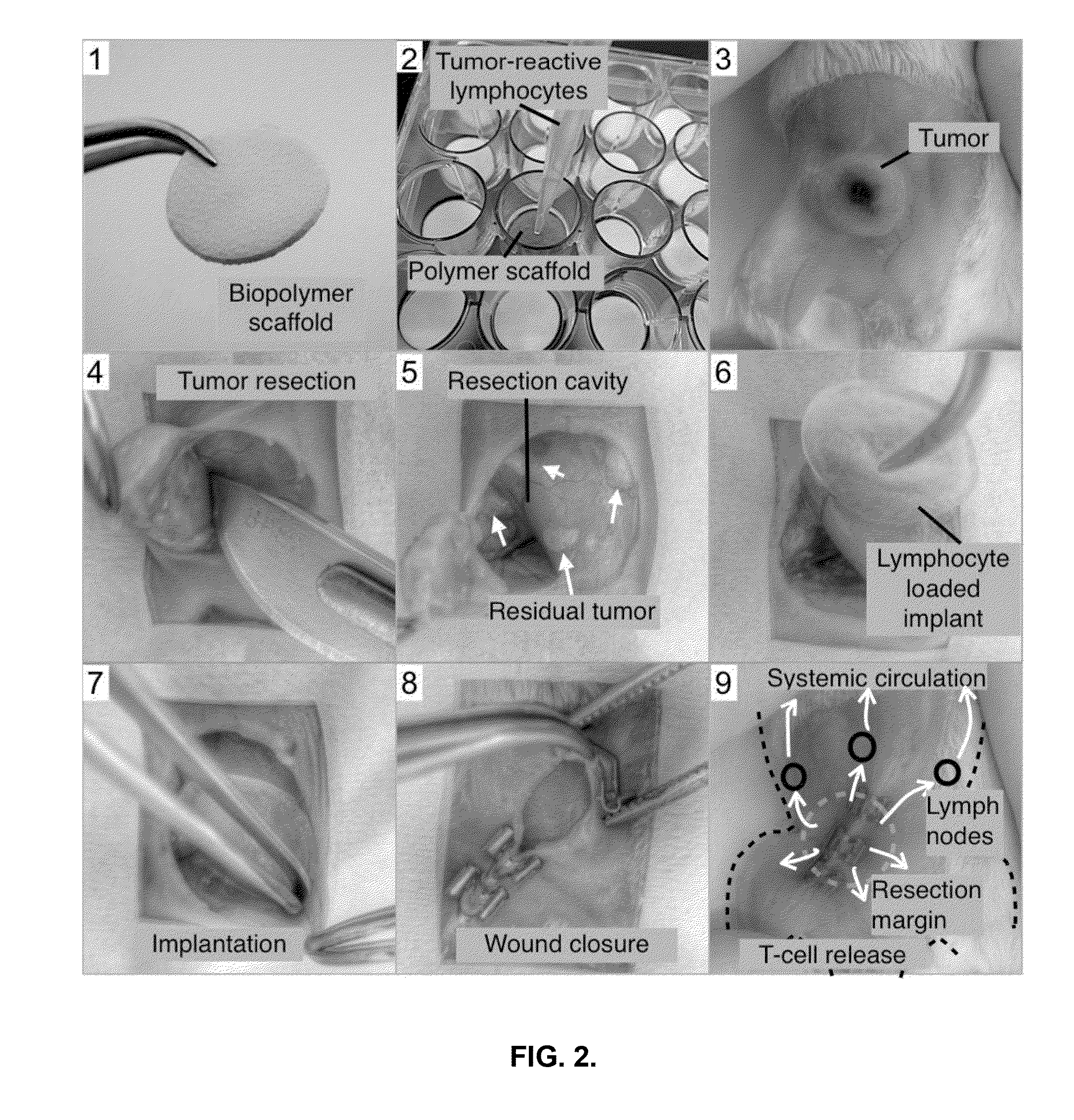
[0133]To model clinical ACT, in which tumor-reactive T-cells are isolated from patients and expanded in the laboratory, an established protocol to obtain breast tumor-specific T-cells from BALB / c mice (Restifo, Nat Rev Immunol 12, 269-281 (2012) which is incorporated by reference herein for its teachings regarding the same) was optimized. First, a 4T1 mammary carcinoma cell line that expresses the costimulatory ligands B7.1 and 4-1 BBL was generated using retroviral vectors (FIGS. 4A and 4B). This genetic modification helps the immune system recognize 4T1 tumor antigens as foreign. Irradiated 4T1 B7.1 / 4-1 BBL (hereafter 4T1-STIM) cells act as a whole cell cancer vaccine and prime tumor-specific T-cells in BALB / c mice following tail-base injection (4×106 tumor cells). To enhance further vaccine-driven immune responses, the adjuvants CpG oligodeoxynucleotide and poly(I:C) were mixed with 4T1-STIM cells before i...
example 2
Tumor-Reactive T-Cells Injected Intravenously or Locally into the Tumor Resection Bed Fail to Prevent Tumor Relapse Due to Inefficient Tumor Homing and / or Poor Persistence
[0134]Whether standard intravenous injections of 4T1 tumor-reactive T-cells could reduce cancer relapse emanating from incompletely excised 4T1 tumor was assessed. The chosen 4T1 breast tumor model very closely mimics the tumor growth and metastatic spread of human breast cancer to lymph nodes, liver, lung, and bone. Tumor cells, retrovirally tagged with Gaussia luciferase for bioluminescence imaging, are easily transplanted into the right mammary gland of BALB / c mice and develop tumors that are ˜10 mm in size after two weeks. At that time point, mice were preconditioned for the adoptive transfer of T-cells by removing homeostatic cytokine sinks by lymphodepletion (250 mg / kg cyclophosphamide, injected intraperitoneally). The following day, tumors were resected, leaving behind 0.1-1% residual disease as quantified b...
example 3
Porous Polysaccharide Scaffolds Coated with Collagen-Mimetic Peptide Support Rapid “Lymph Nodelike” Motility and Sustain the Viability of Embedded T-Cells
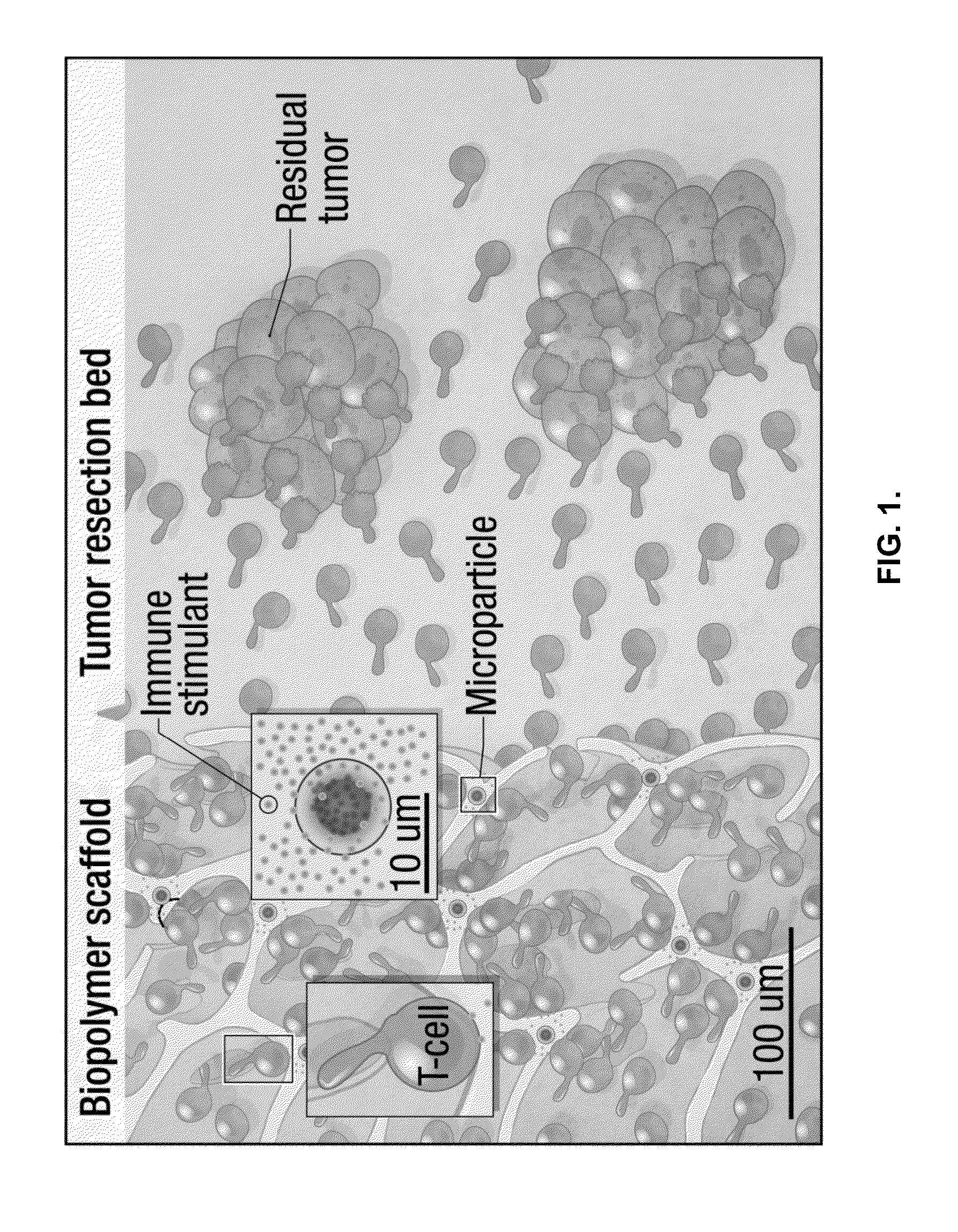
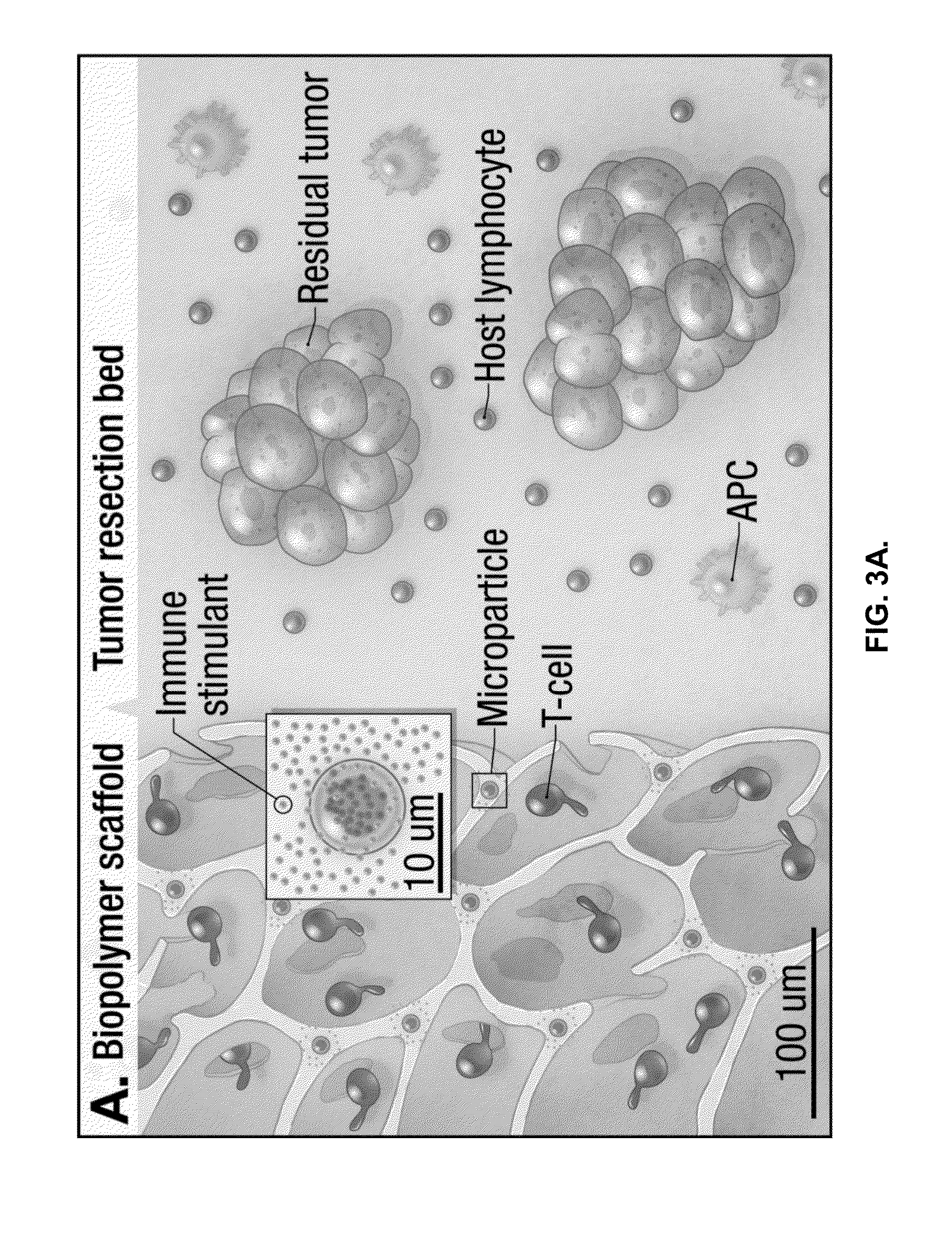
[0137]To address the issues noted above, implantable compositions were created to produce a new microenvironment at the tumor resection site conducive to the sustained proliferation of transferred lymphocytes. The compositions can be used to deliver tumor-reactive lymphocytes to residual tumor following resection while sustaining their effector function and survival. To function as a lymphocyte delivery and release platform, a composition needs to provide sufficient mechanical support for embedded cells, a cell-adhesive coating to enable loaded cells to migrate through the material and exit into tissue, and appropriate stimulatory signals to trigger cell proliferation.
[0138]As an example, under physiological conditions, T-cells migrate in peripheral tissue along collagen fibers. Whether anchoring the collagen-mimetic peptide GFOGER...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com