Low swell tissue adhesive and sealant formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0116]The present invention is further defined in the following Examples. It should be understood that these Examples, while indicating preferred embodiments of the invention, are given by way of illustration only. From the above discussion and these Examples, one skilled in the art can ascertain the essential characteristics of this invention, and without departing from the spirit and scope thereof, can make various changes and modifications of the invention to adapt it to various uses and conditions.
[0117]Reagent Preparation
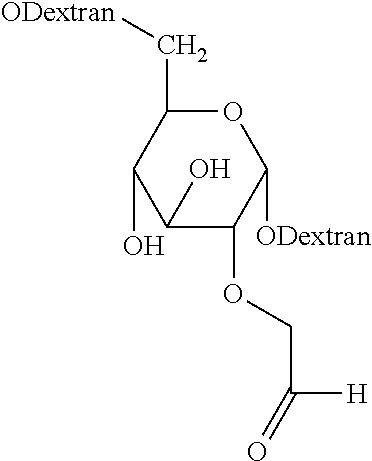
[0118]Preparation of Dextrans Having Pendant Aldehyde Groups (AFD-15-177-15% and AFD-10-216-94%).
[0119]Dextran containing pendant aldehyde groups and having a weight-average molecular weight of about 10 kDa to about 15 kDa, an equivalent weight per aldehyde group of about 177, and a degree of aldehyde substitution of about 115% was prepared using a two-step procedure. In the first step, dextran having an average molecular weight of about 8.5-11.5 kDa was reacte...
examples 1-5
Low Swell Hydrogels
[0136]The purpose of these Examples was to demonstrate the low swell property of the hydrogels disclosed here.
[0137]Hydrogels were formed by mixing two aqueous solutions, the first aqueous solution containing the aldehyde-functionalized dextran (AFD-15-177-115%) and 300 ppm of FD&C Blue #1 dye, and the second aqueous solution containing a polyethylene glycol amine (P8-10-1). The two aqueous solutions were mixed using a dual barrel syringe with a 16 stage mixing tip (MEDMIX SYSTEMS AG, Rotkreuz, Switzerland). The concentrations of the aqueous solutions used are given in Table 1. The resulting mixture was injected directly into a piece of plastic tubing having an internal diameter of about 4 mm. After gelation, the hydrogel was removed from the tubing by slitting the tubing open. The resulting cylindrical hydrogel was cut into pieces having a length of approximately 10 mm and each piece was placed inside a separate piece of plastic tubing, which had an internal diam...
example 6
In Vivo Adhesion Prevention in a Rabbit Laminectomy Model
[0141]The compositions of the present disclosure were tested to determine their effectiveness in preventing the formation of deleterious fibrous tissues in a Rabbit Laminectomy study. Two low swell compositions (i.e., Examples 1 and 5) were evaluated for adhesion prevention in a rabbit dorsal laminectomy model.
[0142]Aqueous solutions of AFD-15-177-115% (10 wt % and 15 wt %) and P8-10-1 (15 wt / o) were prepared and sterilized as follows. The appropriate weight of each component was calculated and adjusted for moisture content (as measured using a Mettler Toledo HB43-S moisture analyzing balance). This amount was weighed into a glass vessel and the calculated weight of water was added. A volume of a stock solution of FD&C Blue #1 dye was added to each of the AFD-15-177-115% solutions to give a final concentration of 300 ppm. The resulting solutions were capped and placed on a shaker incubator at 170 rpm and 40° C. until the compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com