5-ala for detection of brain tumors
a brain tumor and 5-ala technology, applied in the field of brain tumor detection methods, can solve the problems of inability to adequately distinguish radionecrosis from mri imaging, complex treatment and risk definition, and inability to accurately detect radionecrosis, etc., and achieve high polydispersity index, high polydispersity index, and high polydispersity index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Detection and Characterization of Blood-Derived Microparticles from 5-ALA Treated GBM Patients
[0064]This Example demonstrates detection of a small molecule drug following oral dosing. The drug can be uptaken by tumor cells, enzymatically modified, and shed back into circulating microparticles within hours of dosing. Furthermore, the enzymatic modification of the small molecule drug in the tumor cells was detectable by fluorescence from blood-derived microparticles.
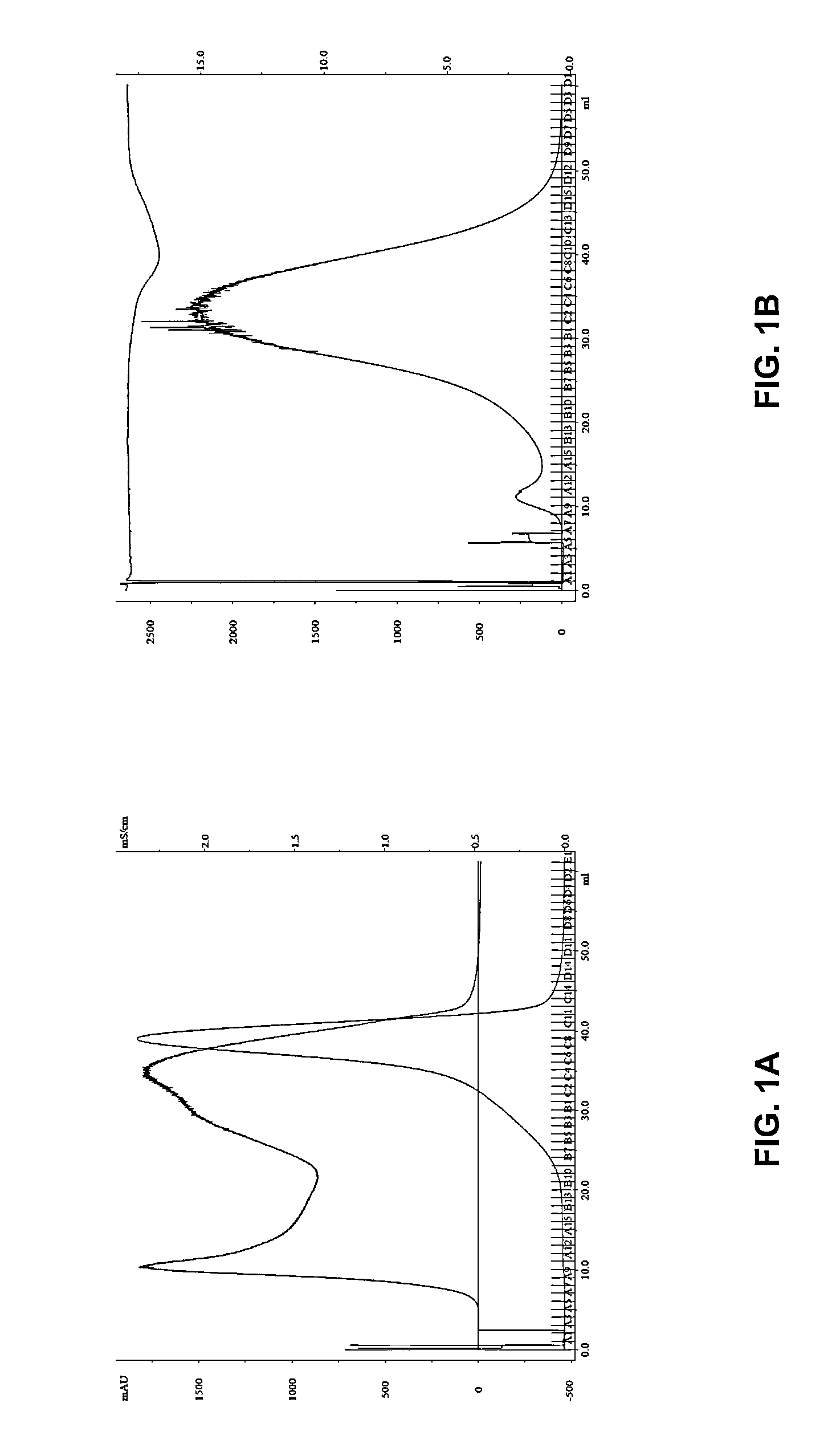
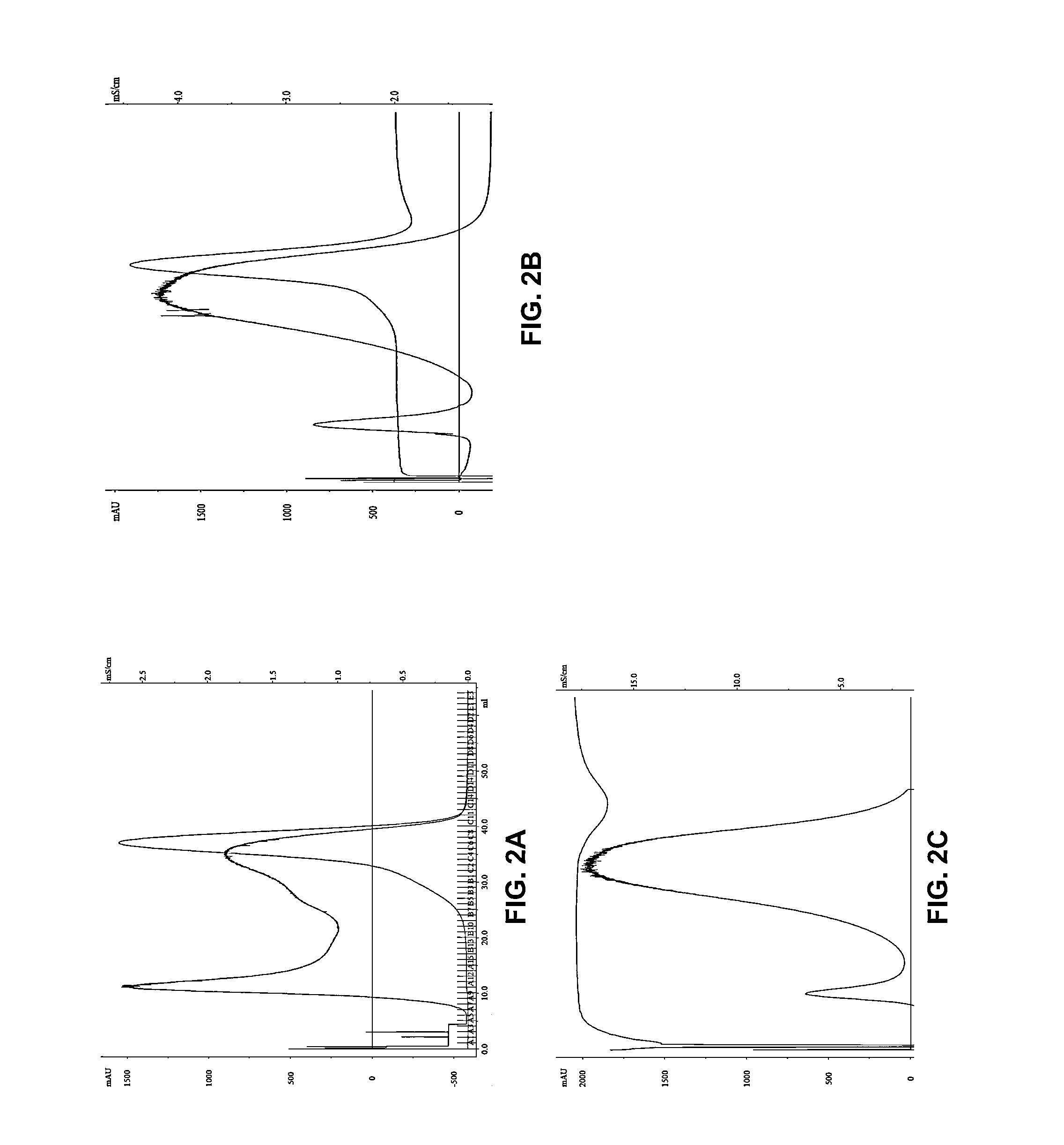
[0065]The purpose of this study was to (i) isolate nanometer-diameter microparticles from the serum of glioblastoma (GBM) patients that have taken Gliolan™ PO, (ii) quantify their number using two different isolation methods (ddH2O and PBS-based chromatography) and (iii) assess whether or not these microparticles contain detectable levels of endogenous fluorescence when excited with a UV laser.
[0066]Gliolan® (5-aminolevulinic acid hydrochloride) is currently approved in Europe for the intraoperative visualization of malign...
example 2
U87 Cell Line Converts 5-ALA to PPIX Via a CPOX-Mediated Conversion Process which can be Monitored in Shed Microparticles
[0136]This example demonstrates that U87 cell cultures actively convert 5-ALA to PPIX, that these cells produce coproporphyrinogen III oxidase (CPOX protein), and that CPOX proteins and PPIX are present in shed microparticles in cancer cell lines.
In Vitro Studies in U87
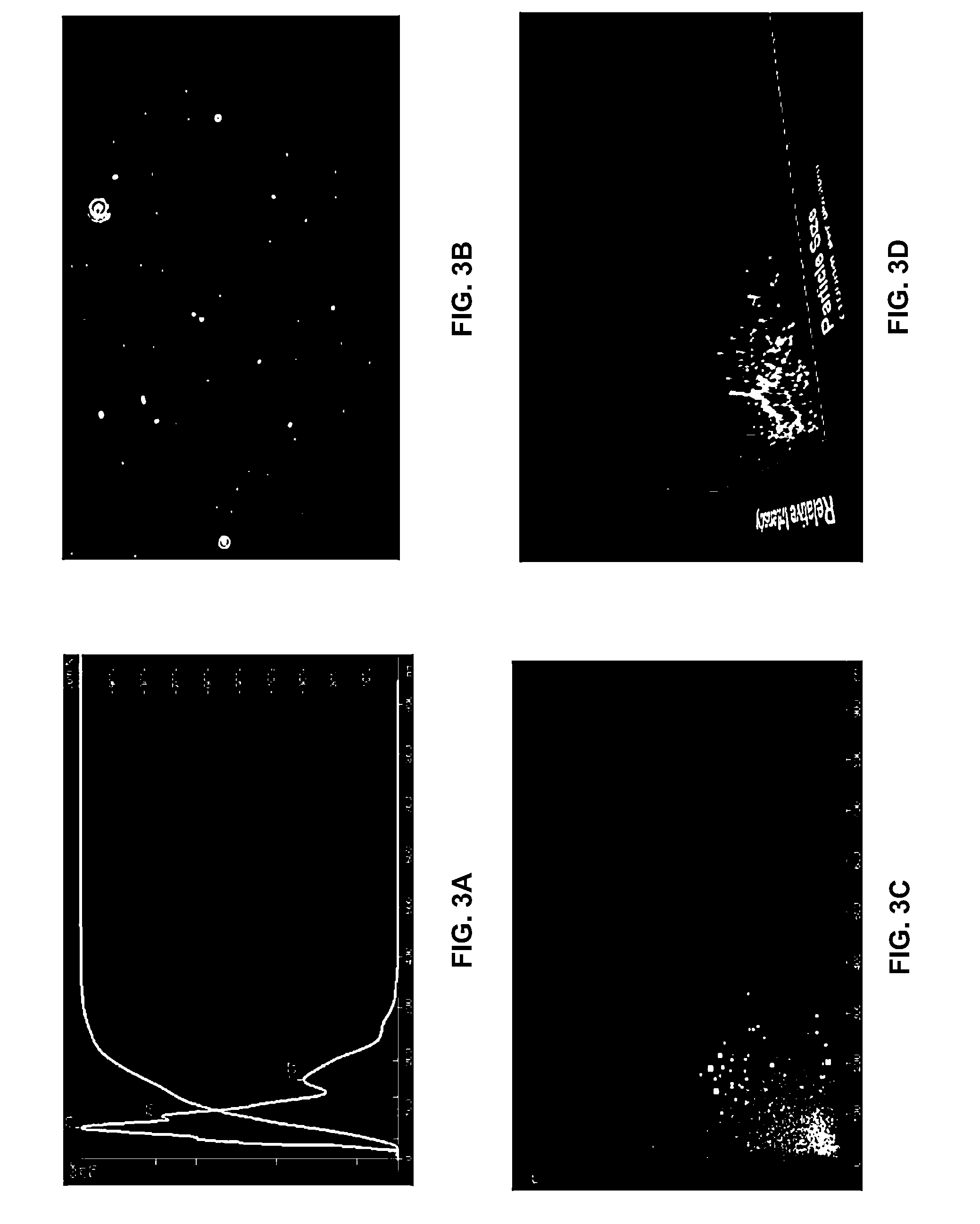
[0137]U87 cells representative of GBM tumors grown in T-75 flasks, in the absence of serum, were exposed to 5-aminolevulinic acid (5-ALA; 250 μg / ml) for 4 or 24 hours. Following exposure, cells were subjected to fluorescent excitation in a LSRII flow cytometer with an excitation at 406 nM violet wave length. Filter specifications were set at either 450 / 50, or set at 610 / 20 for the detection of protoporphyrin IX (PPIX).
[0138]It was found that the mean fluorescence index (MFI), as a function of time, did not change in the absence of 5-ALA. In contrast, MFI increased following exposure to 5-ALA observe...
example 3
Detection of GBM by Administration of Gliolan™
[0142]A patient is treated for GBM by surgical resection, external beam radiation, and temozolomide.
[0143]Per hospital standard protocol, the patient is evaluated on a regular basis (monthly) for clinical symptoms of disease recurrence with observational and MRI evaluation for GBM recurrence.
[0144]Gliolan™ (5-ALA) is administered at a dosage of 20 mg / kg three hours prior to the time scheduled for the blood draw.
[0145]Blood is drawn and allowed to coagulate to produce serum or spun at 1000 g for 5 minutes to produce plasma. The biological fluid is frozen at −80 C and preserved for later processing. Microparticles are isolated using size exclusion chromatography with 2% agarose as the solid phase. Double distilled H2O, (100% v / v) or PBS is used as mobile phase with a serum or plasma load volume <2% of bed volume and elution separation for molecular weight entities validated with protein standards.
[0146]5-ALA-mediated fluorescence associate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com