Ocular Compositions and Methods Thereof
a technology of compositions and therapeutic agents, applied in the direction of aerosol delivery, transferases, peptide/protein ingredients, etc., can solve the problems of difficult or impractical treatment of numerous diseases and conditions that affect the eyes, including those for which therapeutic agents are available, and achieve the effect of increasing the ocular residence time and bioavailability of therapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
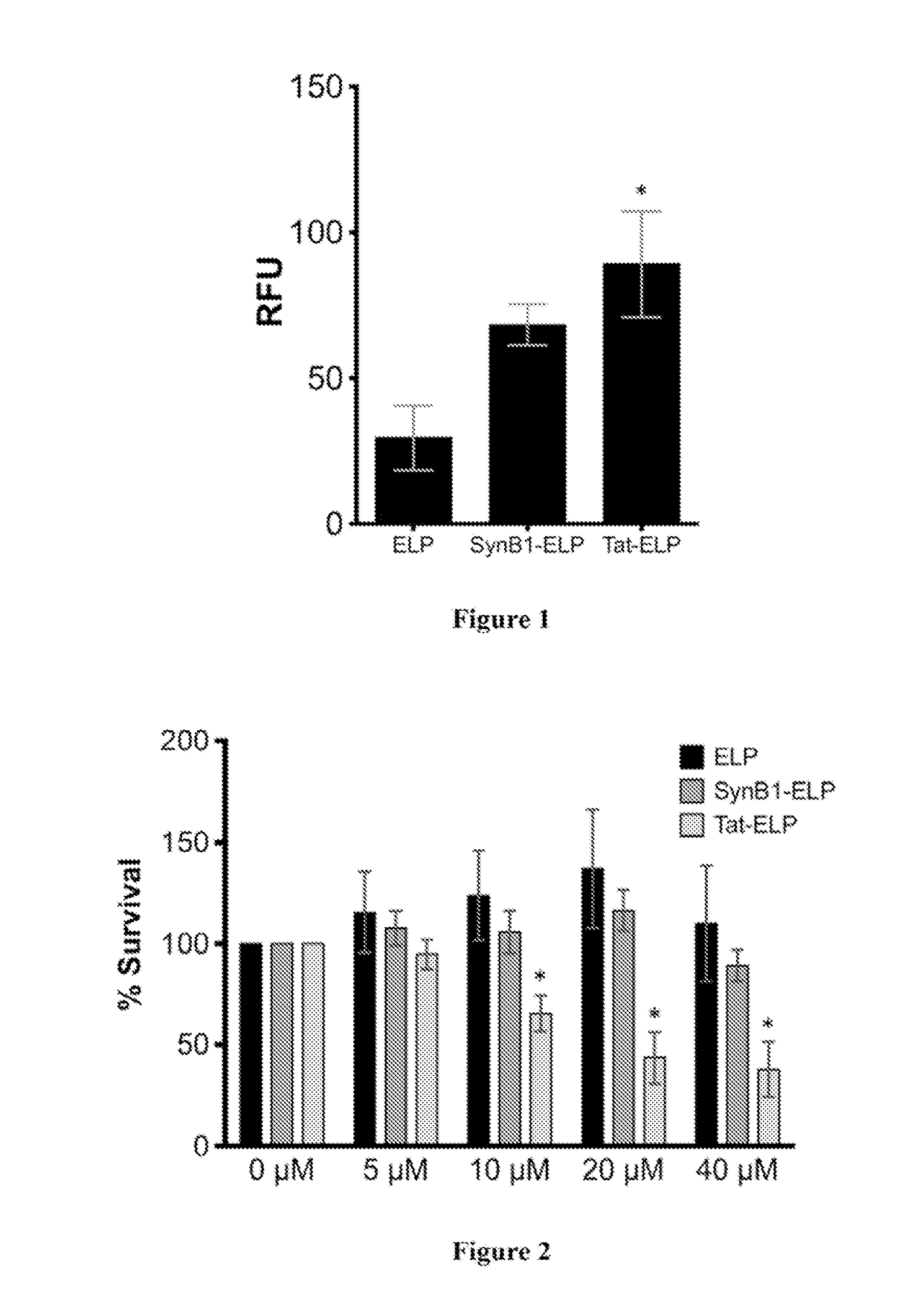
[0072]This Example characterizes the uptake of elastic-like polypeptides (ELPs) and cell-penetrating peptide ELP fusion polypeptides (CPP-ELPs) in human corneal epithelial cells (HCEs). To determine if CPPs could mediate the uptake of ELP in corneal cells, HCEs grown in culture were exposed to 10 μM fluorescently-labeled ELP, SynB1-ELP, or Tat-ELP. After 24 h incubation with the labeled proteins, the cells were detached and analyzed by flow cytometry. The mean fluorescence intensity was determined for all cells, and the fluorescence value was corrected to account for differences in labeling efficiency among the proteins. As shown in FIG. 1, ELP was detectable over autofluorescence in HCE cells, and the cellular uptake was increased 2.8-fold and 3.9-fold with SynB1 and Tat CPPs, respectively.
[0073]In addition to uptake efficiency, toxicity of ELP or CPP-ELPs to HCE cells was examined. Cells were exposed to varying concentrations of ELP, SynB1-ELP, or Tat-ELP for 72 hours, and cell nu...
example 2
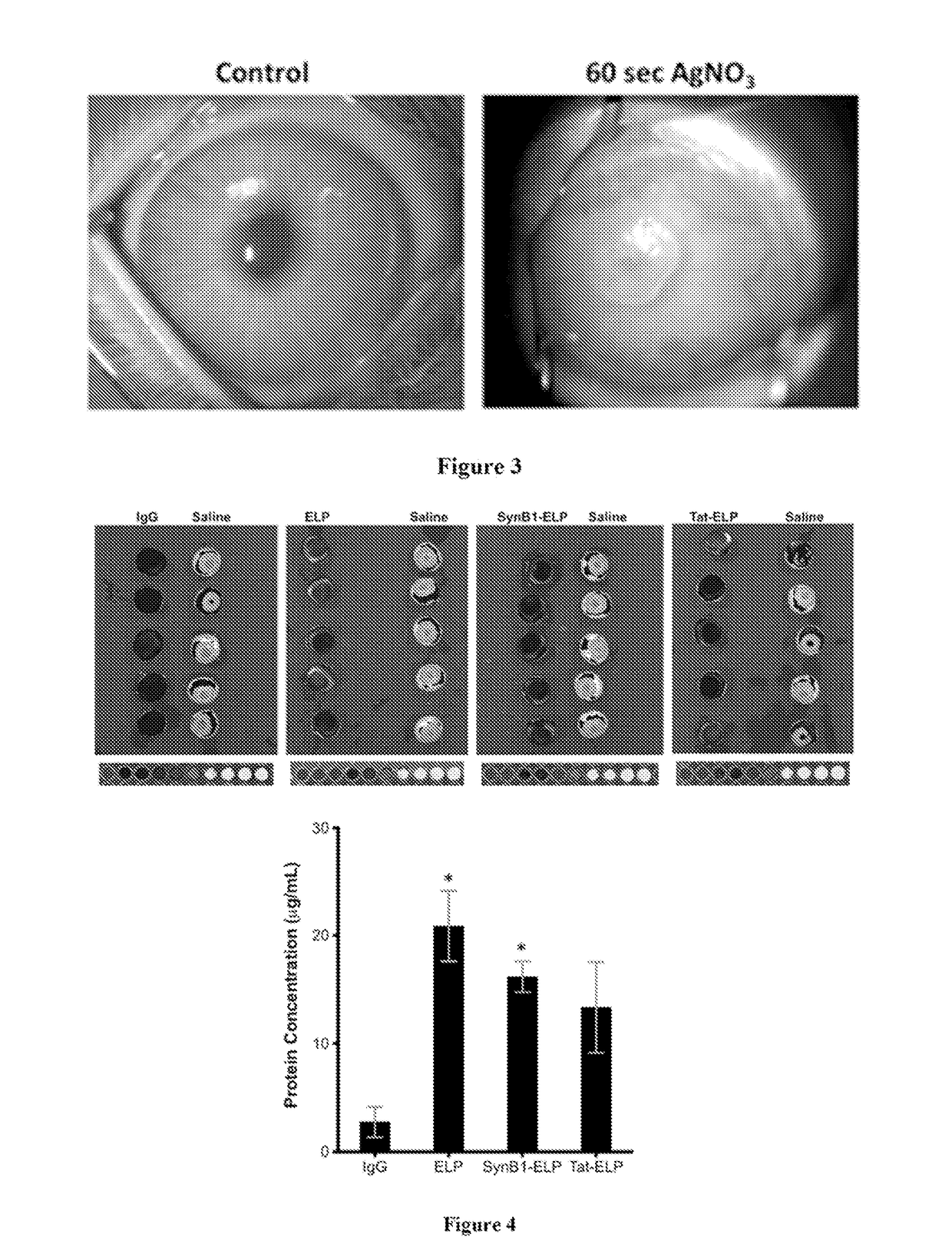
[0074]This Example characterizes the development of a rabbit corneal neovascularization (CN) model. Rabbits were chosen for this model because the thickness of their corneal epithelial layer is similar to that of humans. New Zealand white rabbits were anesthetized with isoflurane, and a corneal burn was induced using a 60 second application of a silver nitrate cautery stick. As shown in FIG. 3, 7 days after corneal injury, the rabbits developed a neovascular response in the injured eye.
example 3
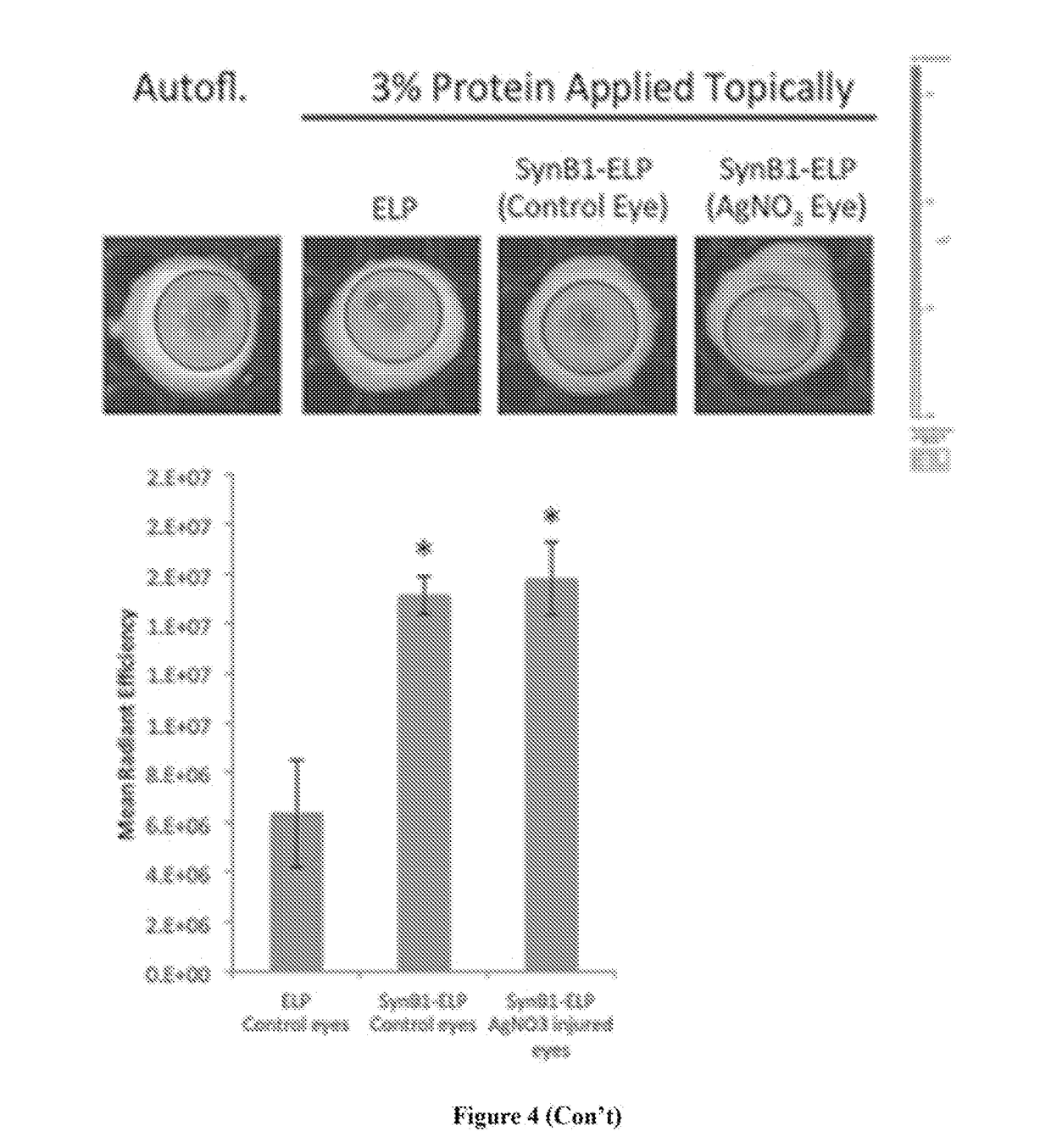
[0075]This Example characterizes penetration of the corneal barrier by ELP and CPP-ELPs, and compares ELP corneal accumulation to a model antibody, immunoglobulin. G (IgG). Fluorescently labeled ELP, SynB1-ELP, Tat-ELP, or IgG was applied topically via eye drops three times over 6 h in rabbits. The contralateral eye was administered saline control. 8 h after the first application, the animals were sacrificed and the eyes removed for ex vivo analysis. As shown in FIG. 4, ELP accumulated in the rabbit cornea at levels over seven-fold higher than IgG. SynB1-ELP and Tat-ELP also accumulated in the cornea much more efficiently than IgG, but the CPP-ELP corneal levels were not enhanced relative to ELP control.
[0076]After total fluorescence analysis, the eyes were frozen and cut using a cryomicrotome. Sagittal sections were used in order to visualize the cornea in cross-section. Sections were stained with DAPI to mark cell nuclei and imaged with an epifluorescence microscope. As shown in F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com