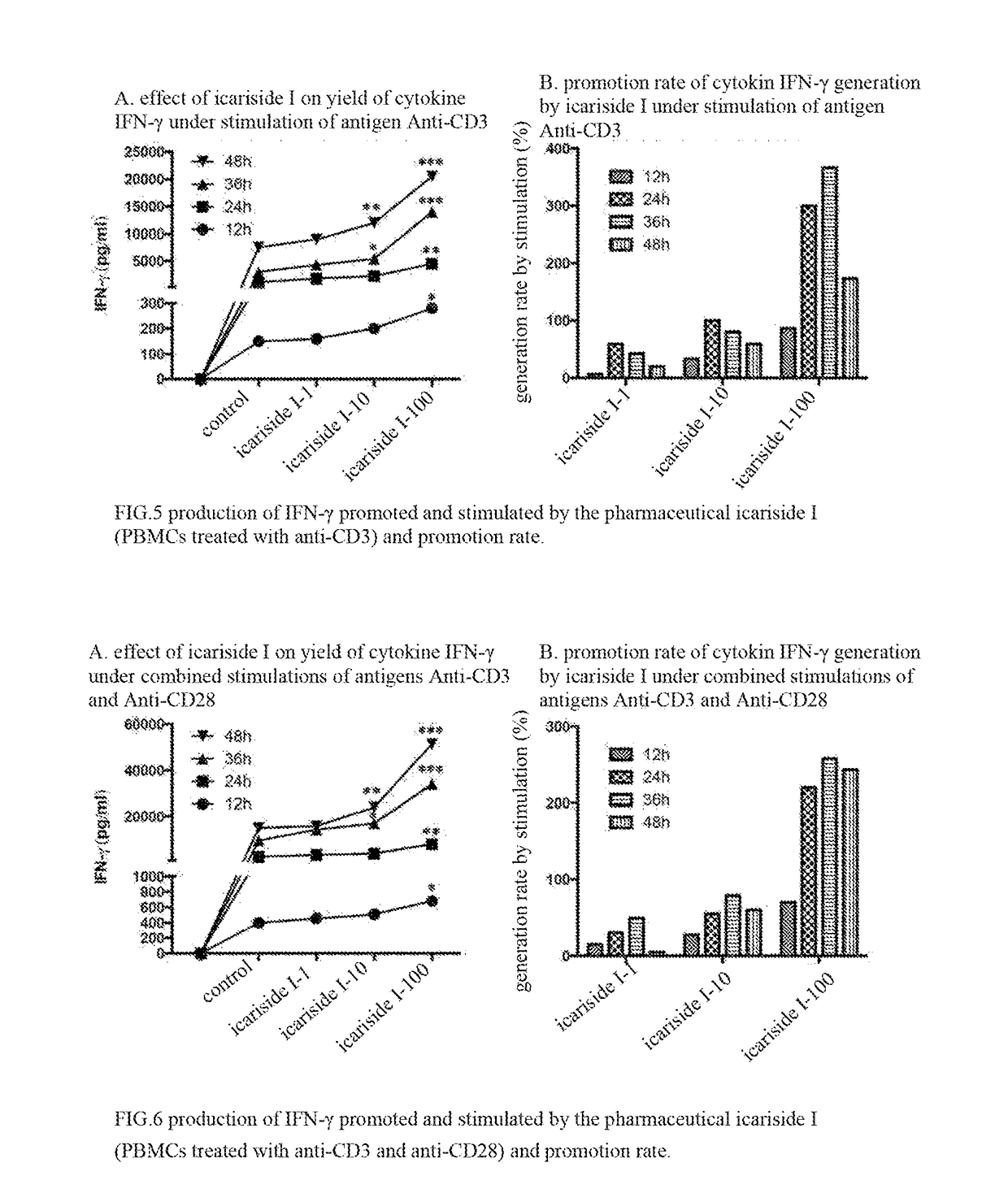
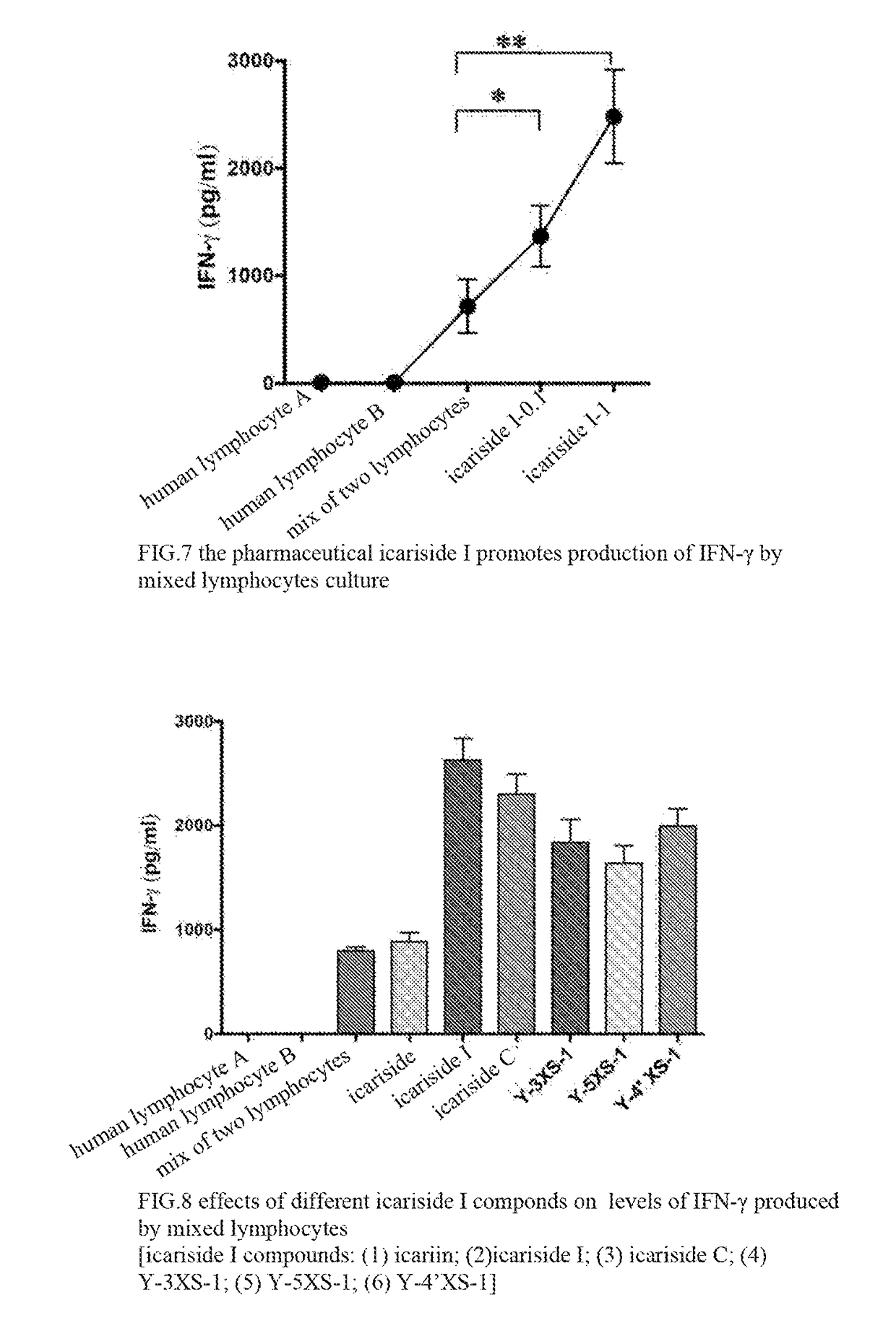
Icariside compound, preparation method thereof, and application thereof
a technology of icariside and compound, applied in the field of icariside compound, can solve the problems of limiting application, easy degradation, people getting sick, etc., and achieve the effects of improving or inhibiting human immunity, low toxicity and side effects, and safe us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
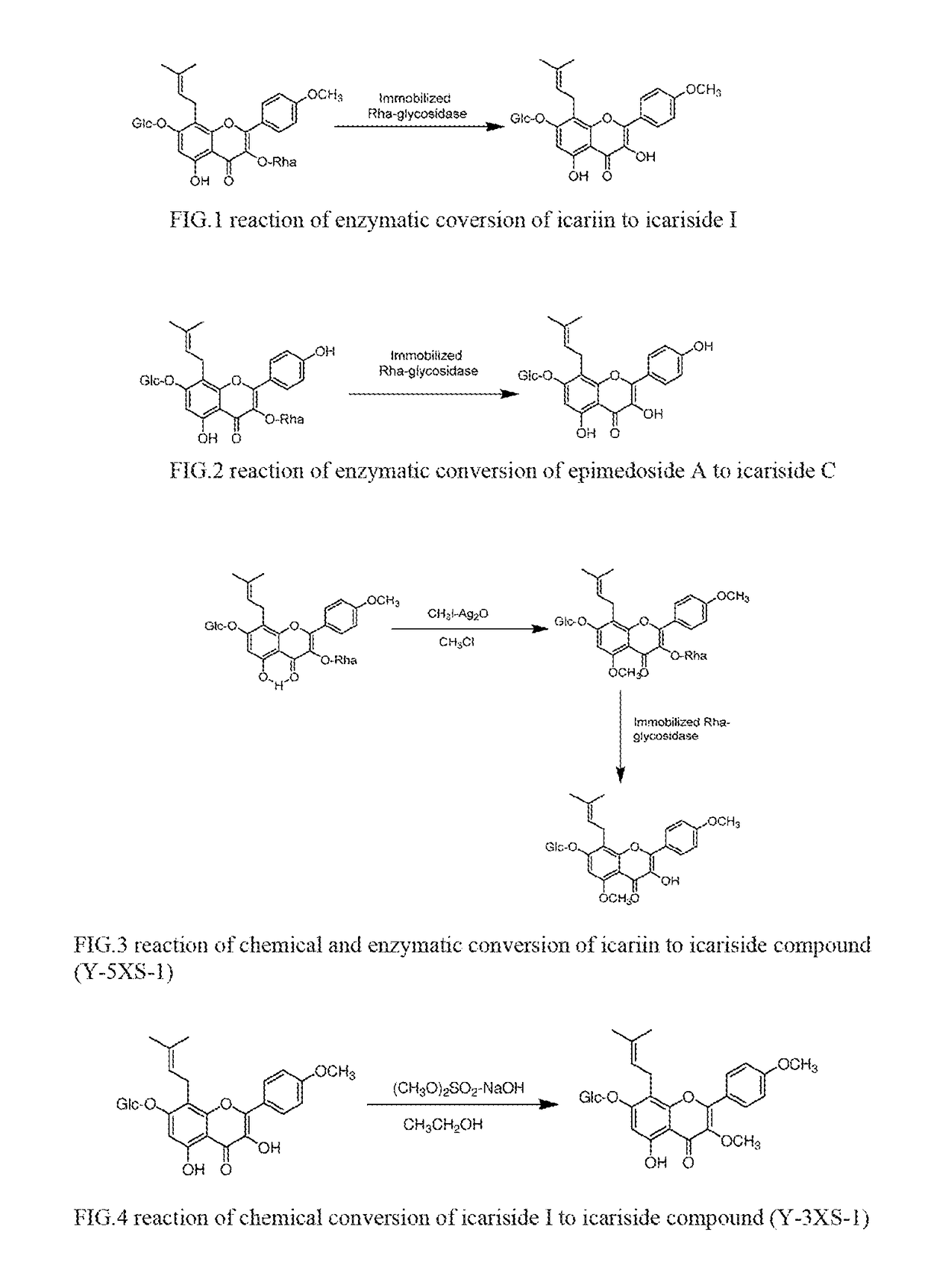
[0035]Preparation of Icariside I
[0036](1) Preparation of Icariside I by Separation from Epimedium.
[0037]According to the method reported by Li Wenkui, et al. (Herbal, 1995, 26 (9), 453-455), icariside I was separated and purified with a content of more than 95% from the whole plant of epimedium. The chemical structure of the product was characterized by 1H NMR, Mass spectroscopy, and other instrumentations.
[0038](2) Preparation of Icariside I by Enzymatic Conversion of Icariin as Raw Material.
[0039]A. Preparation of icariin: According to the method reported by Li Wenkui, et al., (Herbal, 1995, 26 (9), 453-455), icariin was separated and purified with a content of more than 95% from the whole plant of epimedium. The chemical structure of the product was characterized by 1H NMR, Mass spectroscopy, and other instrumentations.
[0040]B. Preparation of icariside I:
2 g of icariin (purity 98%) was dissolved in a pH6.8 phosphate buffer, and then 1 g of immobilized rhamnosidase was added. The ...
example 2
[0041]Preparation of Icariside C
[0042](1) Preparation of Icariside C by Separation from Epimedium.
[0043]According to the method reported by Li Wenkui, et al. (Herbal, 1995, 26 (9), 453-455), icariside C with a content of more than 95% was obtained after separation and purification from the whole plant of epimedium. The chemical structure of the product was characterized by 1H NMR, Mass spectroscopy, and other instrumentations.
[0044](2) Preparation of Icariside I from Epimedoside A as Raw Material by Enzymatic Conversion.
[0045]A. Preparation of epimedoside A: epimedoside A is one of the components with a higher content in epimedium. According to the method reported by Xu Sui Xu et al. (Herbs, 1981, 14, 24-26), epimedoside A with a content of more than 95% was obtained by separation and purification from the whole plant of epimedium. The chemical structure of the product was characterized by 1H NMR, Mass spectroscopy, and other instrumentations.
[0046]B. Preparation of icariside C: Ica...
example 3
[0047]Preparation of Icariside Compounds Based on Modification of the R1 Group
[0048]Using icariin as raw material, the OH group of R1 was selectively transformed into another group by a chemical method (the process does not affect glycosyl groups present in the molecule). Then, by applying the method in Example 1 (2), the R2 Rha group of the modified icariin was removed with an enzymatic method to obtain the desired icariside compounds.
[0049]Preparation Example: 2 ml chloroform was added to 50 mg icariin followed by addition of 10 mg iodomethane (CH3I) and addition of 10 mg silver oxide (Ag2O). Then, the solution was stirred at room temperature for 24 hours. After reaction, chloroform was removed by rotary evaporation. The residue was re-dissolved with 95% ethanol and was purified by column chromatography (silica gel column) to obtain the product with R1 being a methoxy group. Finally, by applying the method in Example 1 (2), the R2 Rha group in the modified icariin was removed with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com