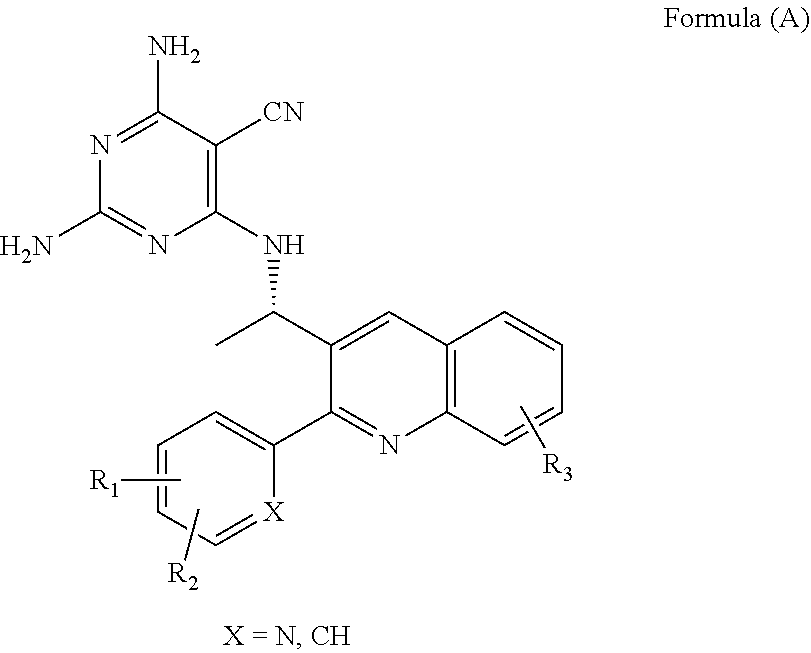
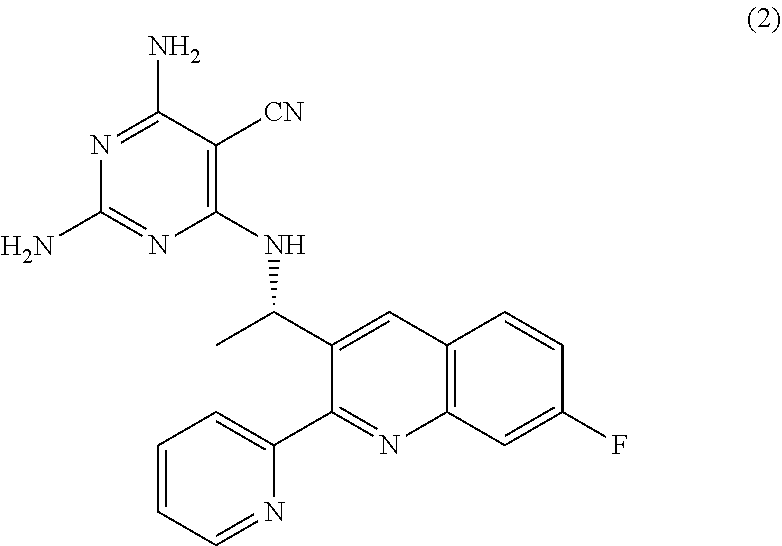
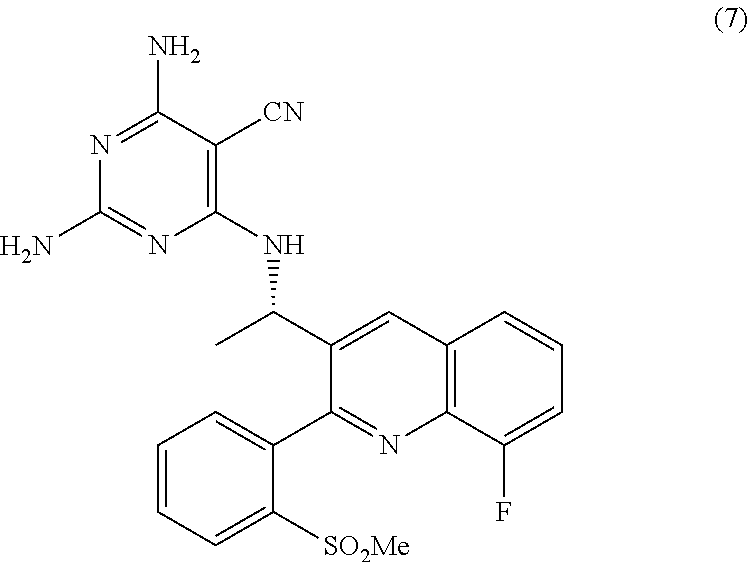
Quinoline analogs as phosphatidylinositol 3-kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fluoro-2-pyridin-2-yl-quinolin-3-yl)-ethylamine (9)
[0065]Step 1:
[0066]To a solution of 2-chloro-8-fluoroquinoline-3-carboxaldehyde (1.5 g, 7.2 mmol) in anhydrous THF (20 mL) was added titanium isopropoxide (4.3 mL, 1.4 mmol) at r.t. After 15 minutes, (R)-2-methyl-2-propanesulfinamide (0.867 g, 7.2 mmol) was added and stirring was continued overnight at r.t. Water (100 mL) was added to the reaction mixture and the precipitate obtained was filtered and washed with DCM. The organic layer was dried (Na2SO4), filtered and concentrated in vacuo to give the crude material as a pale yellow solid which was purified by column chromatography on silica gel (EtOAc / hexane, 4 / 5)) to give a pale yellow solid (2.0 g, 89%). Mass Spectrum (ESI) m / e: 313 (M+1).
[0067]Step 2:
[0068]To a solution of 2-Methyl-propane-2-sulfinic acid 2-chloro-8-fluoro-quinolin-3-ylmethyleneamide (0.95 g, 2.8 mmol) in DCM (22 mL) was added dropwise MeMgCl (1.94 mL, 5.8 mmol; 3 M in THF) over 10 minutes at −78° C. under nitrog...
example 2
Fluoro-2-phenyl-quinolin-3-yl)-ethylamine (12)
[0074]
[0075]Compound (S)-2-(1-(2-Chloro-7-fluoroquinolin-3-yl)ethyl)isoindoline-1,3-dione was prepared according to the literature (J. Med. Chem. 2015, 58, 480-511). This compound (280 mg, 0.79 mmol), phenylboronic acid (146 mg, 1.2 mmol), and potassium carbonate (328 mg, 2.4 mmol) were combined in 6 mL of anhydrous DMF under an atmosphere of N2. The solution was purged with N2 for ˜5 min before adding PdCl2(dppf)DCM (64 mg, 0.079 mmol). The solution was heated at 100° C. for 3 h, and then cooled to 50° C. The solution was concentrated under vacuum to give a brownish residue, which was diluted with EtOAc (12 mL). The organic layers were then washed with water (3×3 mL), followed by brine (10 mL). The combined aq. layers were extracted with DCM (3×2 mL). The combined organic layers were dried over MgSO4 and then concentrated under vacuum. The residue obtained was purified by silica gel flash chromatography eluting with a gradient of 20% to...
example 3
fluoro-2-(pyridin-2-yl) quinolin-3-yl)ethanamine (13)
[0078]
[0079]A mixture of (S)-2-(1-(2-chloro-7-fluoroquinolin-3-yl)ethyl)isoindoline-1,3-dione (0.86 g, 2.4 mmol), Pd(PPh3)4 (0.28 g, 0.24 mmol, 0.1 eq) and 2-(tributylstannyl)-pyridine (1.07 g, 2.9 mmol, 1.2 eq) in dioxane (30 mL) was heated to 90° C. under N2. After stirring overnight, LC-MS showed 30% completion. The reaction mixture was heated to 101° C. for additional 2 days. The reaction mixture was then cooled to rt and the resulted solid was filtered and washed with EtOAc to give a tan solid of 2-((S)-1-(7-fluoro-2(pyridin-2-yl)quinolin-3-yl)ethyl)isoindoline-1,3-dione was obtained (0.84 g, 88%). Mass Spectrum (ESI) m / e: 398 (M+1).
[0080]To a slurried suspension of 2-((S)-1-(7-fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)isoindoline-1,3-dione (0.84 g, 2.1 mmol) in anhydrous ethanol (5 mL) was added NH2NH2 (0.34 g, 10.4 mmol) dropwise. The reaction mixture was heated to 90° C. for 30 min and cooled to rt. The reaction mixture w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com