Process of producing monoterpenes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0087]The present invention will now be further described with reference to the following figures which show:
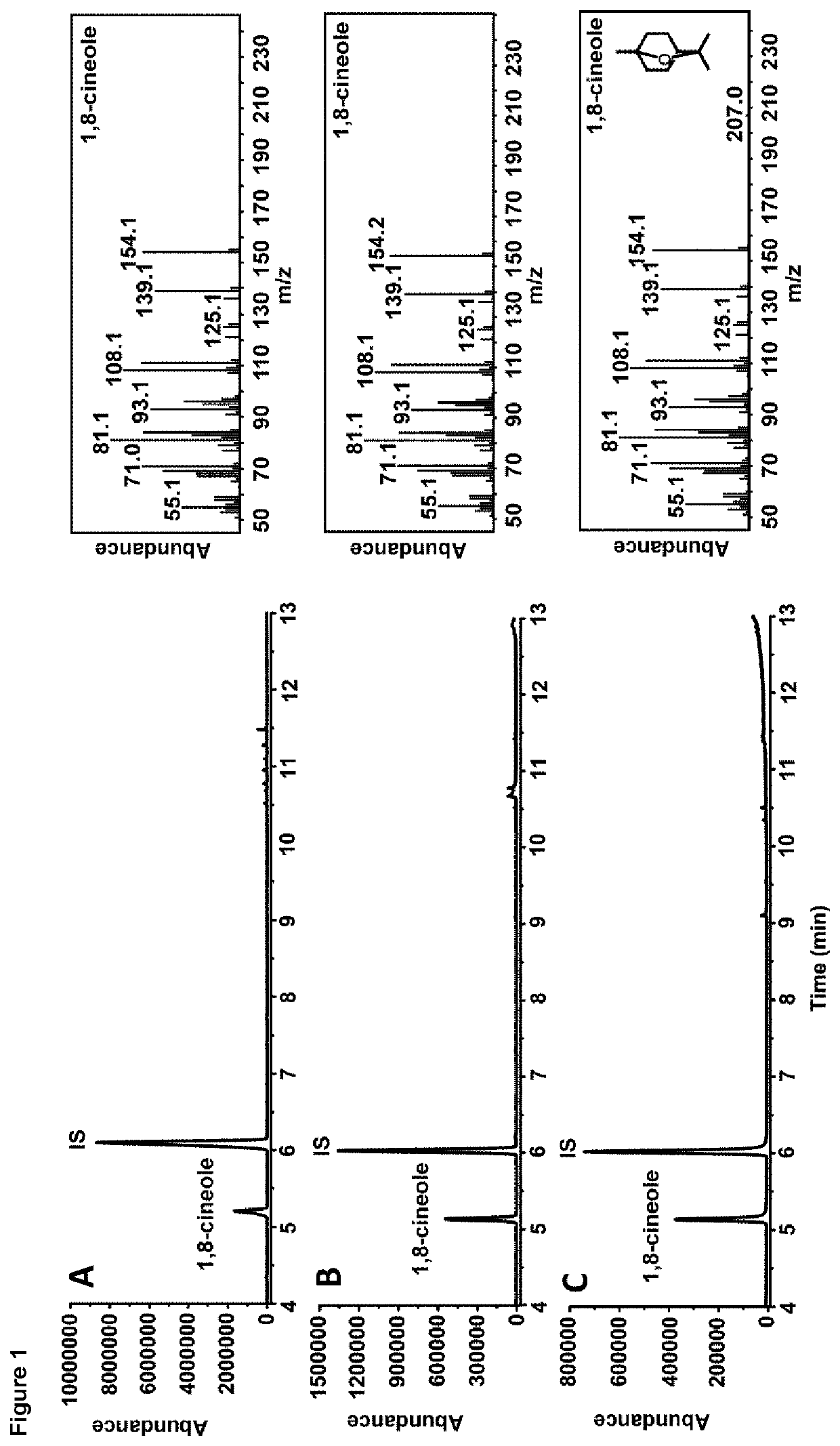
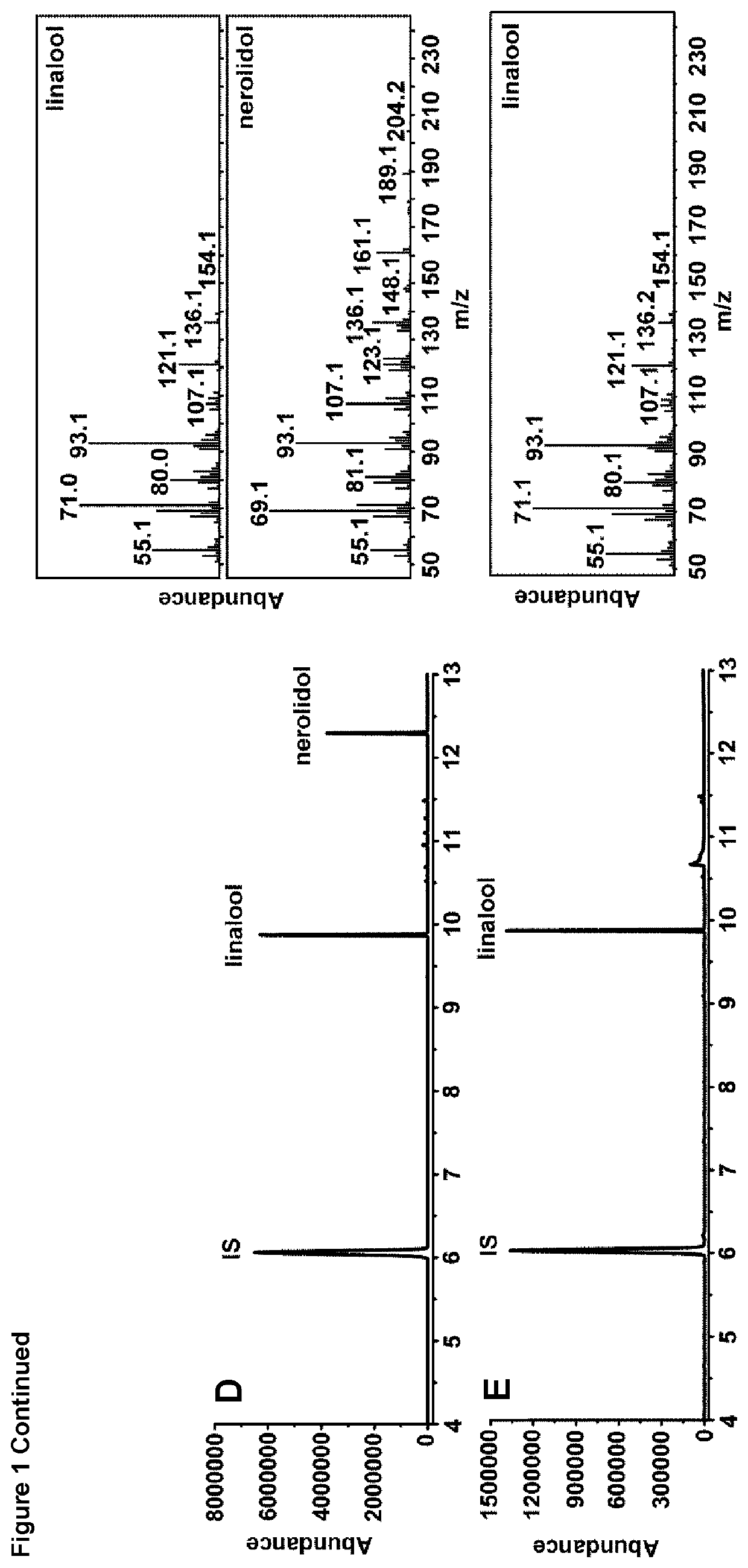
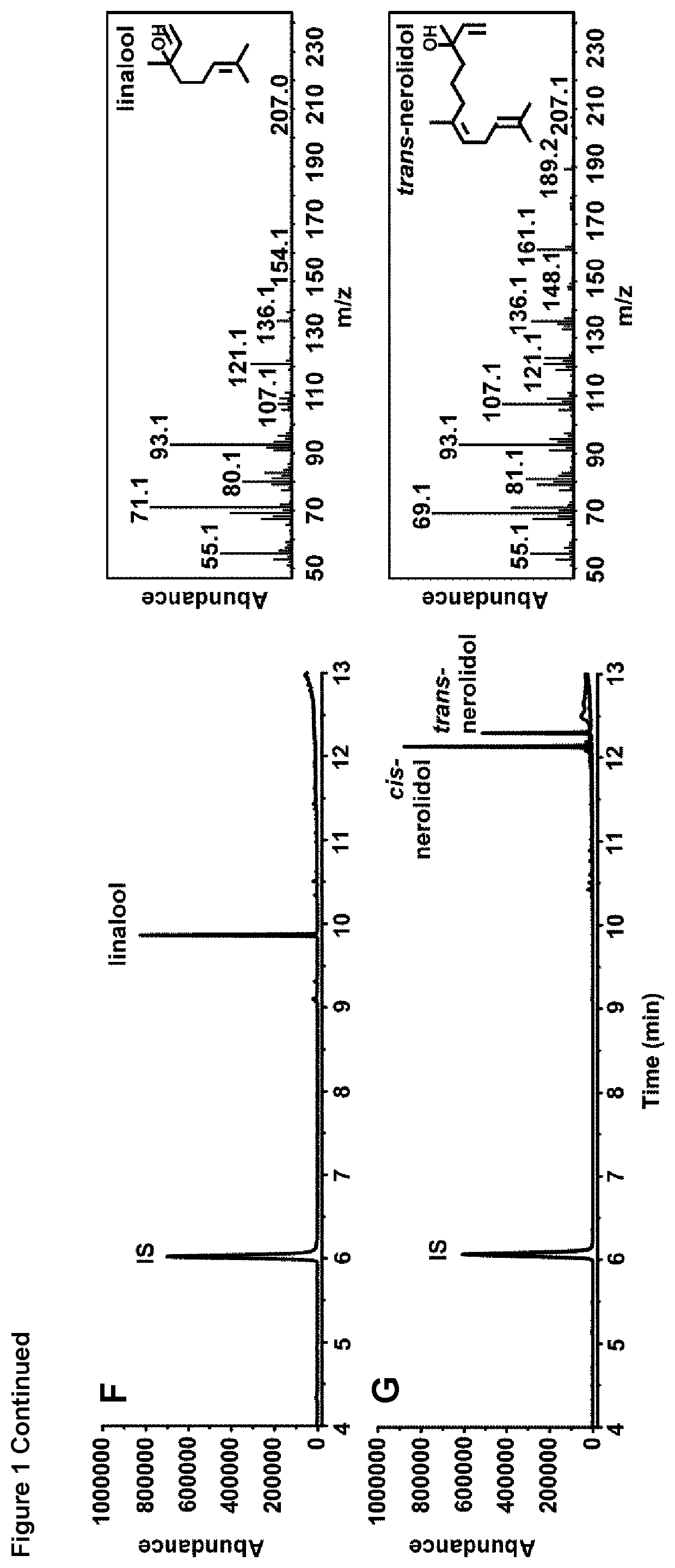
[0088]FIG. 1: GC-MS analysis using bacterial 1,8-cineole synthase (bCinS) and bacterial linalool synthase (bLinS). A) bCinS platform product profile. B) bCinS conversion of GPP (20 mM). C) 1,8-cineole standard (0.1 mg / ml). D) bLinS platform product profile. E) bLinS conversion of GPP (20 mM). F) R-linalool standard (0.1 mg / ml). G) cis- and trans-nerolidol standards (0.1 mg / ml). IS=internal standard (sec-butyl benzene).
[0089]FIG. 2: Structure of bCinS-FNPP complex. Superposition of plant cineole synthase (dark) and bCinS (light). The N-terminal and C-terminal domains of the plant CinS are labelled. An arrow indicates the conformational difference in the helix-break motif.
[0090]Materials and Methods
[0091]Expression and Purification of Bacterial 1, 8-Cineole Synthase (bCinS) and Bacterial Linalool Synthase (bLinS)
[0092]The full-length genes coding for 1,8-cineole synthase (WP_00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com