Pharmaceutical or nutraceutical self-emulsifying solid dispersion composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1.1 Preparations of FT Compositions
[0053]By way of example, any of the FT compositions set forth in Table 1 can usefully be prepared to provide the dissolution, bioaccessibility, absorption, and pharmacokinetic analyses. The FT compositions were prepared from the ingredients including 1-20% T3, 1-20% carrier, 1-5% surfactant, 52-94% filler(s), such as isomalt, starch, lactose, cyclodextrin, isomaltodextrin, lactose+isomalt, lactose+maltodextrins, and lactose+cyclodextrins, and 3% binder (Table 1). The oil phase components, including T3 and surfactant, and the solid phase excipients, including carrier and filler, were well mixed separately. The solid phase excipients were added to the oil phase solution and well mixed. The binder was dissolved in a solvent, such as ethanol, and then added to and well mixed with the solid phase. Alternatively, the binder could be directly added to and mixed with the solid phase. The mixture was sieved and dried, and then passed through a mesh.
TABLE 1S...
example 2
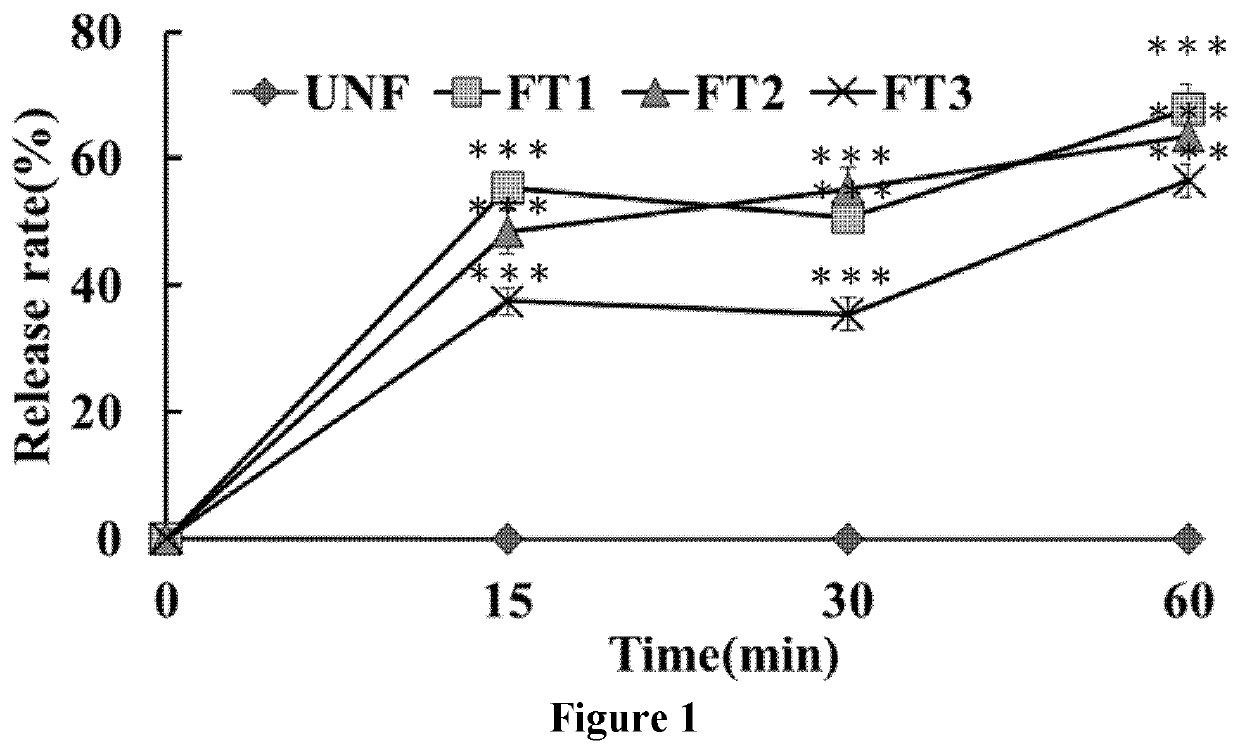
Dissolution Tests of UNF and Various FT Compositions
2.1 Dissolution Test Procedures
[0056]The FT compositions and UNF T3, including 2.0 g formulated powder or 150 mg oil form, were exposed to 750 mL of the acid medium (0.025% SDS-HCl aqueous solution, prepared by dissolving 2.5 g of sodium dodecyl sulfate (SDS) and 83 mL of hydrochloric acid in 10 L of purified water, pH 1.2) followed by exposure to the buffer medium (sequentially add 250 mL of 0.20 M tribasic sodium phosphate to the fluid in the vessel with a total volume was equal to 1000 mL). The dissolution tests were performed in the media with pH 1.2 and 6.8 sequentially. The test solutions in the acid medium were sampled and filtered at 15 and 30 minutes, respectively, through a 0.45 μm syringe filter. After 30 minutes, 250 mL of 0.20 M tribasic sodium phosphate was added to form a buffer medium. Next, the test solutions were sampled after 30 minutes and filtered through a 0.45 μin syringe filter. The collected media were subj...
example 3
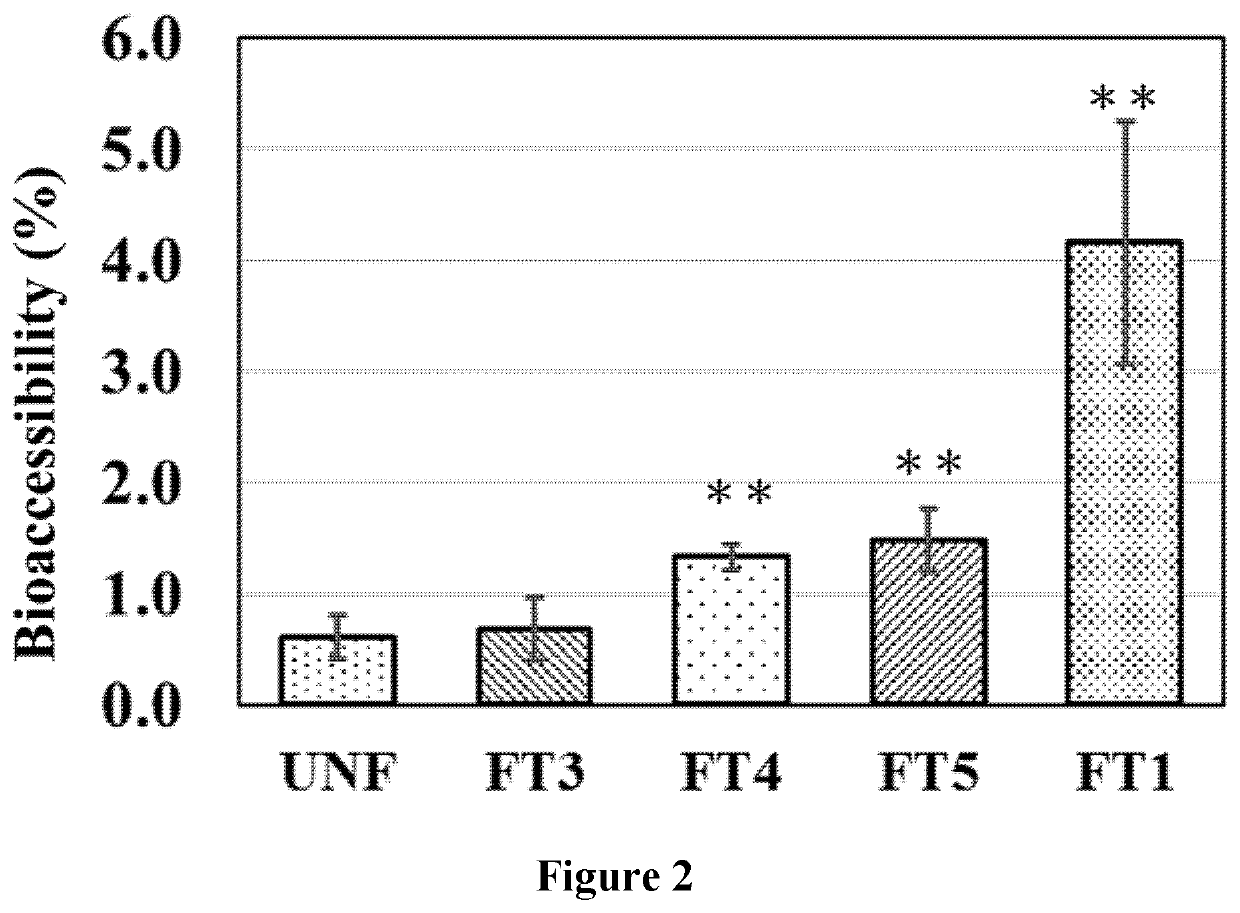
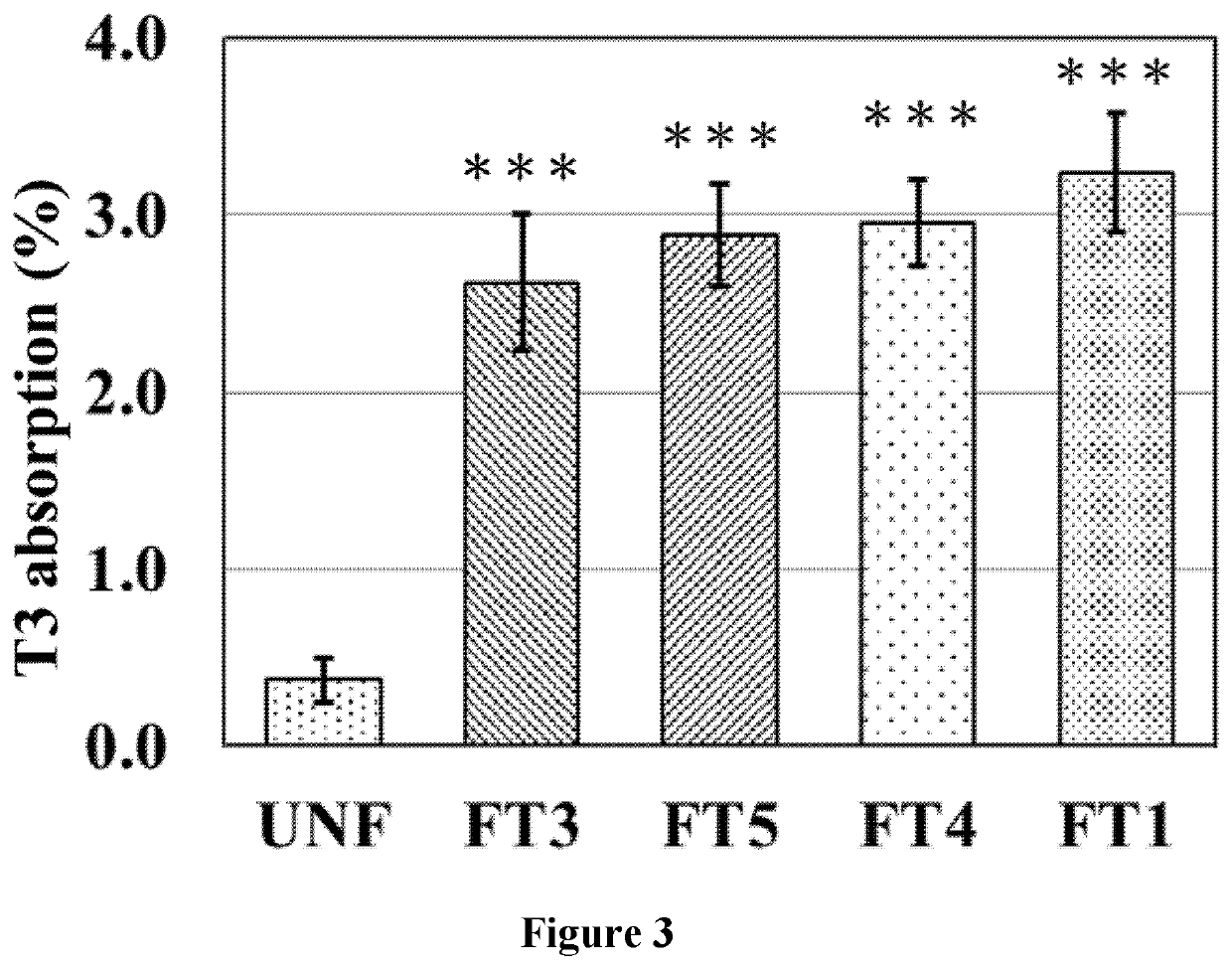
Bioaccessibility (Transcellular Absorption) Analysis Using Caco-2 Cell Model
3.1. Cell Culture
[0058]To investigate the transcellular absorption of T3 by enterocytes, Caco-2 cell model was used. Caco-2 cells were seeded to 0.4 μm transwell insert in 12 well plate. 1 mL of medium was added to the basolateral compartment and 0.5 mL of medium to the apical compartment. The cells were allowed to evenly distribute and attach to the plate button, and incubated at 37° C. with an atmosphere of 5% CO2 and 95% air.
3.2. Differentiation of Caco-2 Cell Lines
[0059]Caco-2 cells started to differentiation spontaneously when the cells reached 80% confluence and after a total culture period of around 14˜21 days they appeared dense microvilli on the apical side, characteristic of small intestinal enterocytes. To ensure the proper differentiation of culture cells, Trans-Epithelial Electrical Resistance (TEER) values were measured. The cultured cells with the values between 250Ω˜400Ω by Ohm Meter were use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap