Hexokinase 2-specific inhibitor in acute central nervous system injury disease
a central nervous system injury and hexokinase inhibitor technology, applied in the field of biomedicine, can solve the problems that both hexokinase 1- and 3-interferences cannot inhibit the activation of hypoxia-induced microglia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
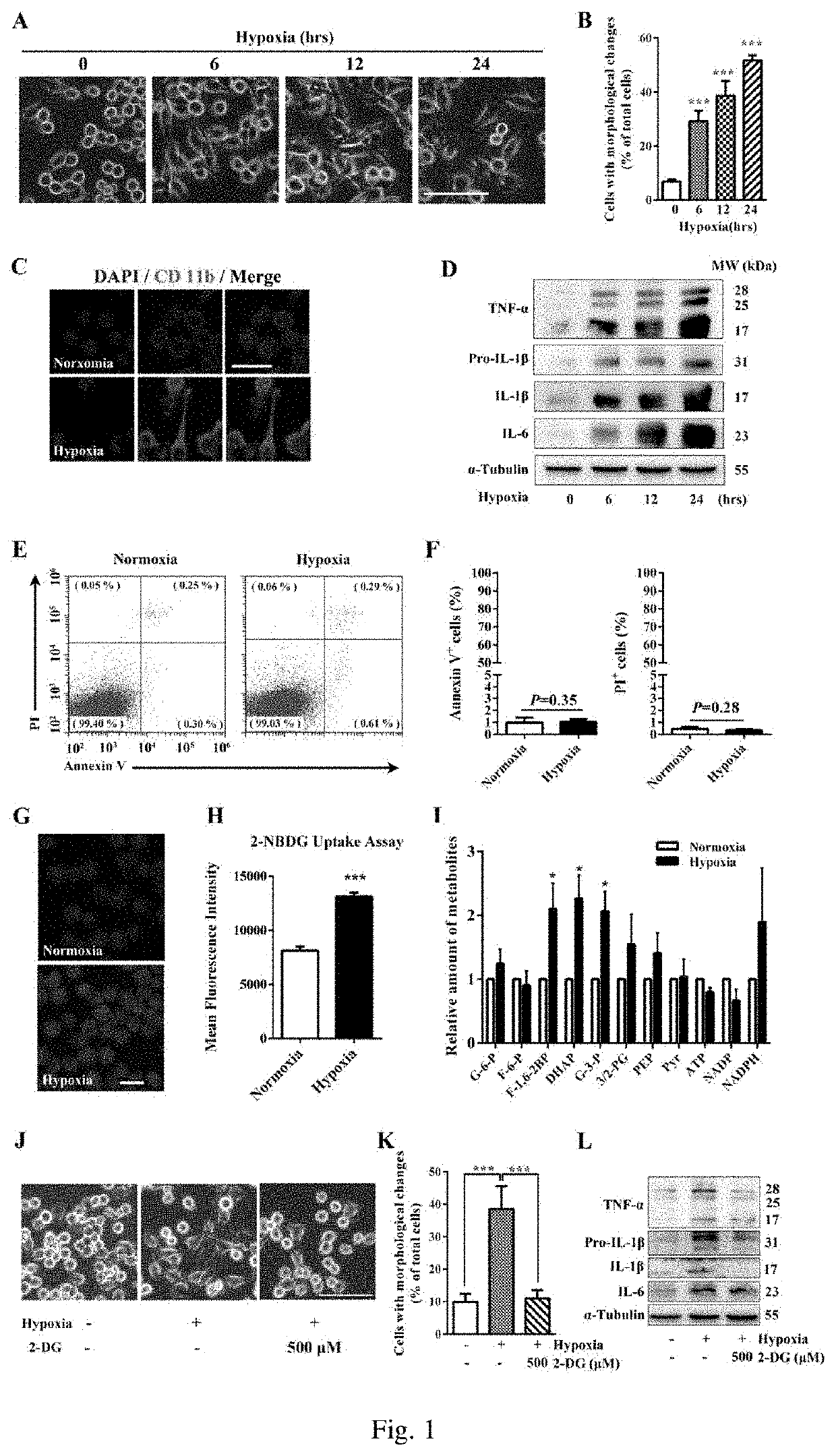
Enhanced Glycolytic Flux is Essential for Hypoxia-Induced Microglial Activation
[0030]Materials
[0031]Mouse BV2 microglia cells, Dulbecco's modified Eagle's medium (DMEM, Gibco, 11965-118), fetal bovine serum (Gibco, 10099-141), 2-deoxy-D-glucose (Sigma-Aldrich, D8375), 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose (2-NBDG, Thermo Fisher Scientific, N13195), Annexin / PI detection kit (Biotool, B32115), flow cytometer (CytoFLEX S), laser scanning confocal microscope (Nikon A1 Spectral Confocal Microscope), anoxic chamber (Coy Laboratory Products).
[0032]Antibodies used in Western blot and immunofluorescence:
[0033]CD 11b antibody (Novus biologicals, NB 110-89474);
[0034]TNF-α antibody (CST, 11498);
[0035]IL-1β antibody (CST, 12507);
[0036]IL-6 antibody (Bioss, bs-6309R);
[0037]α-Tubulin antibody (Bioworld, AP0064).
[0038]Methods
[0039]a) Cell culture: BV2 cells were grown in DMEM containing 10% fetal bovine serum (FBS), placed in 5% CO2, cultured in a 37° C. constant temperat...
example 2
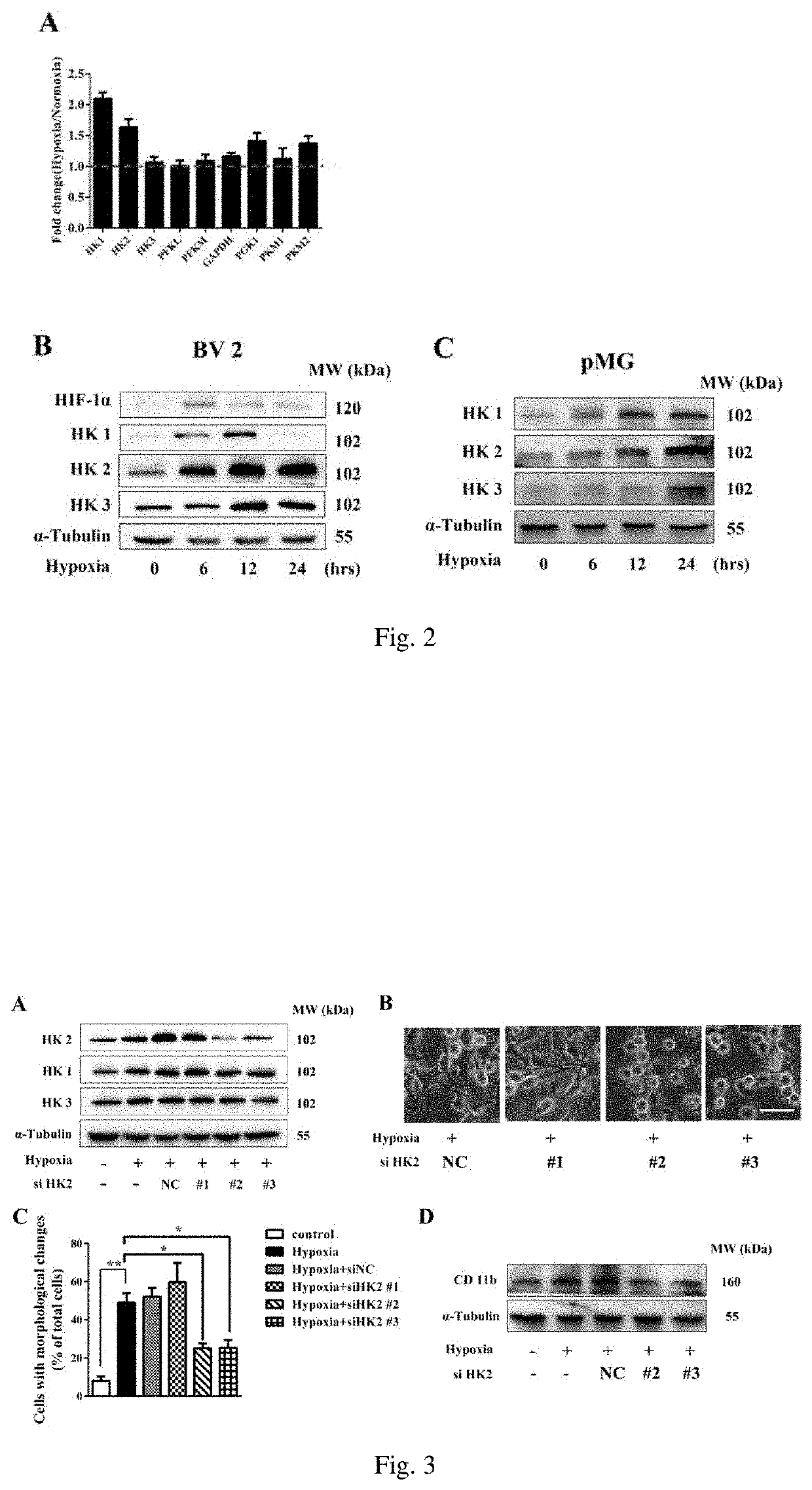
Upregulation of Hexokinase Family Members was Involved in the Microglial Activation After Hypoxia Exposure
[0048]Materials
[0049]Mouse BV2 microglia cell line, primary cultured mouse microglia, high glucose DMEM medium (Gibco, 11965-118), fetal bovine serum (Gibco, 10099-141), RNA extraction reagent TRIzol (Thermo Fisher Scientific, 15596-018), RNA quantification kit (Thermo Fisher Scientific, Q10211), SuperReal qPCR PreMix (SYBR Green) (Tiangen, FP202-01), real-time PCR system (Applied Biosystems), anoxic chamber (Coy Laboratory Products)
[0050]Antibodies used in Western blotting:
[0051]Hexokinase 1 antibody (Abcam,150423),
[0052]Hexokinase 2 antibody (CST, 2867s),
[0053]Hexokinase 3 antibody (Santa Cruz, sc-28890),
[0054]α-Tubulin antibody (Bioworld, AP0064).
[0055]The following mouse gene primer sequences were used in real-time PCR.
PrimersForwardReverseHK1GTAGGGGTACGCTTAGGTGGACCCAGGAGTCCATAAAGCCHK2GAGAAAGCTCAGCATCGTGGTCCATTTGTACTCCGTGGCTHK3GCTCCGTTGAGAGCAGATTTTTGCTGCAAGCATTCCAGTTPFKMGTTT...
example 3
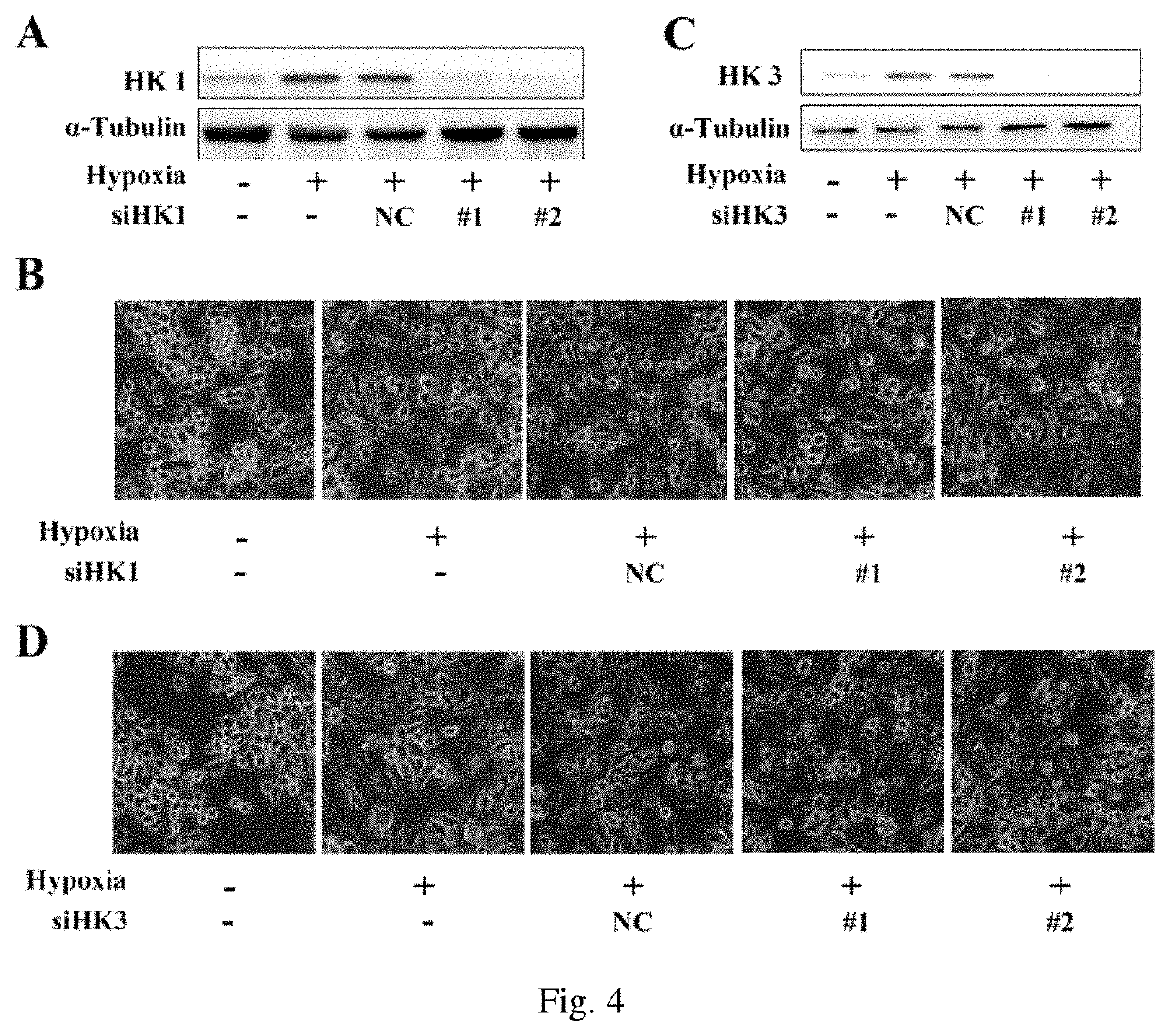
Hexokinase 2, Instead of Other Hexokinase Family Members, Mediated Hypoxia-Induced Activation of Microglia
[0062](1) Hexokinase 2 Interference Could Effectively Inhibit the Hypoxia-Induced Activation Process of Microglia
[0063]Materials
[0064]Mouse BV2 microglia cell line, primary cultured mouse microglia, high glucose DMEM medium (Gibco, 11965-118), fetal bovine serum (Gibco, 10099-141), siRNA fragments, siRNA transfection reagent (Lipofectamine RNAiMAX Reagent, Thermo Fisher Scientific, 13778-500), inverted phase contrast microscope (Nikon ECLIPSE Ti Microscope), laser confocal microscope (Nikon A1 Spectral Confocal Microscope), anoxic chamber (Coy Laboratory Products).
[0065]Antibodies used in Western blotting:
[0066]Hexokinase 1 antibody (Abcam,150423),
[0067]Hexokinase 2 antibody (CST, 2867s),
[0068]Hexokinase 3 antibody (Santa Cruz, sc-28890),
[0069]CD 11b antibody (Novus biologicals, NB 110-89474),
[0070]α-Tubulin antibody (Bioworld, AP0064).
[0071]Methods
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| Phase contrast images | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com