Composition for preventing or treating neurodegenerative diseases, containing diterpene-based compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-b
[0086]4.47 kg of the stem and root of Daphne genkwa were chopped, and then immersed in 12 L 80% ethanol for 4 hours, filtered to separate the solids and a first liquid component. The separated solid was again immersed in 12 L 80% ethanol for 4 hours, and filtered to obtain a second liquid component. The first liquid component and the second liquid component were mixed, the mixture was concentrated under reduced pressure, and the residue was lyophilized to prepare 255.1 g of extract of Daphne genkwa.
Example 2: Separation of Active Ingredient by Various Solvents from the Extract of Daphne genkwa
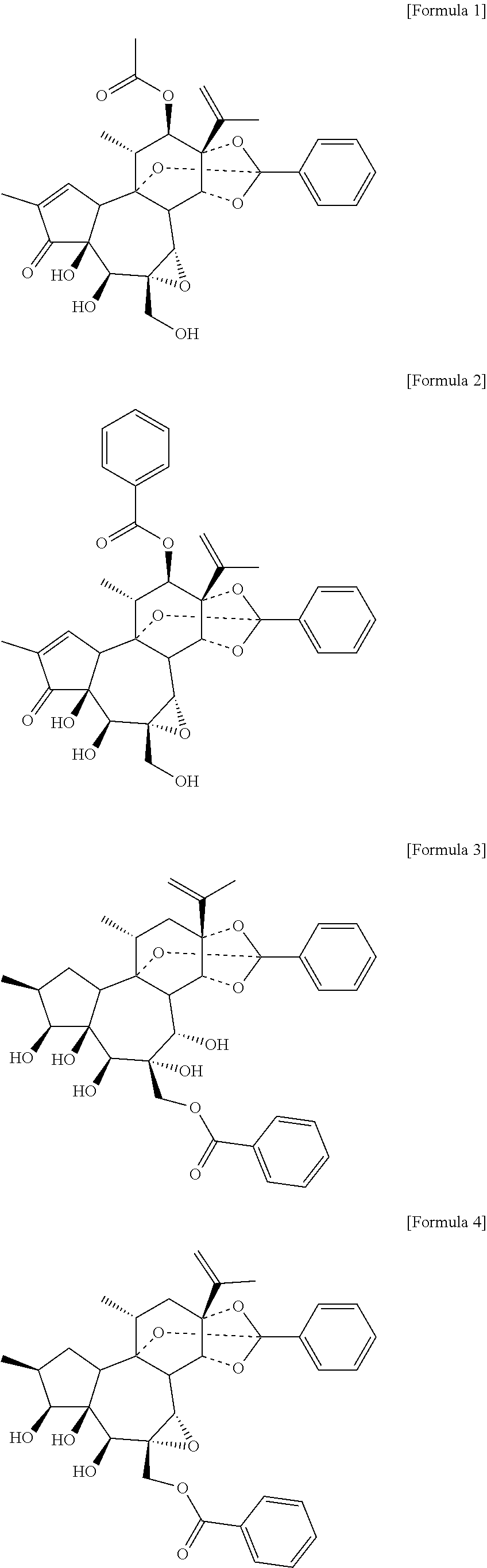
[0087]The extract of the flower of Daphne genkwa obtained in Example 1-a was sequentially fractionated with 2 L of distilled water and 2 L of hexane, chloroform, ethyl acetate, and butanol, respectively. The chloroform layer was concentrated under reduced pressure, and the chloroform fraction (17.6 g) was eluted with a gradient mixed solvent (100:0, 50:1, 20:1, 10:1, 5:1, 2:1, 1:1) of chlorof...
example 3
Compounds on Nurr1 Activity
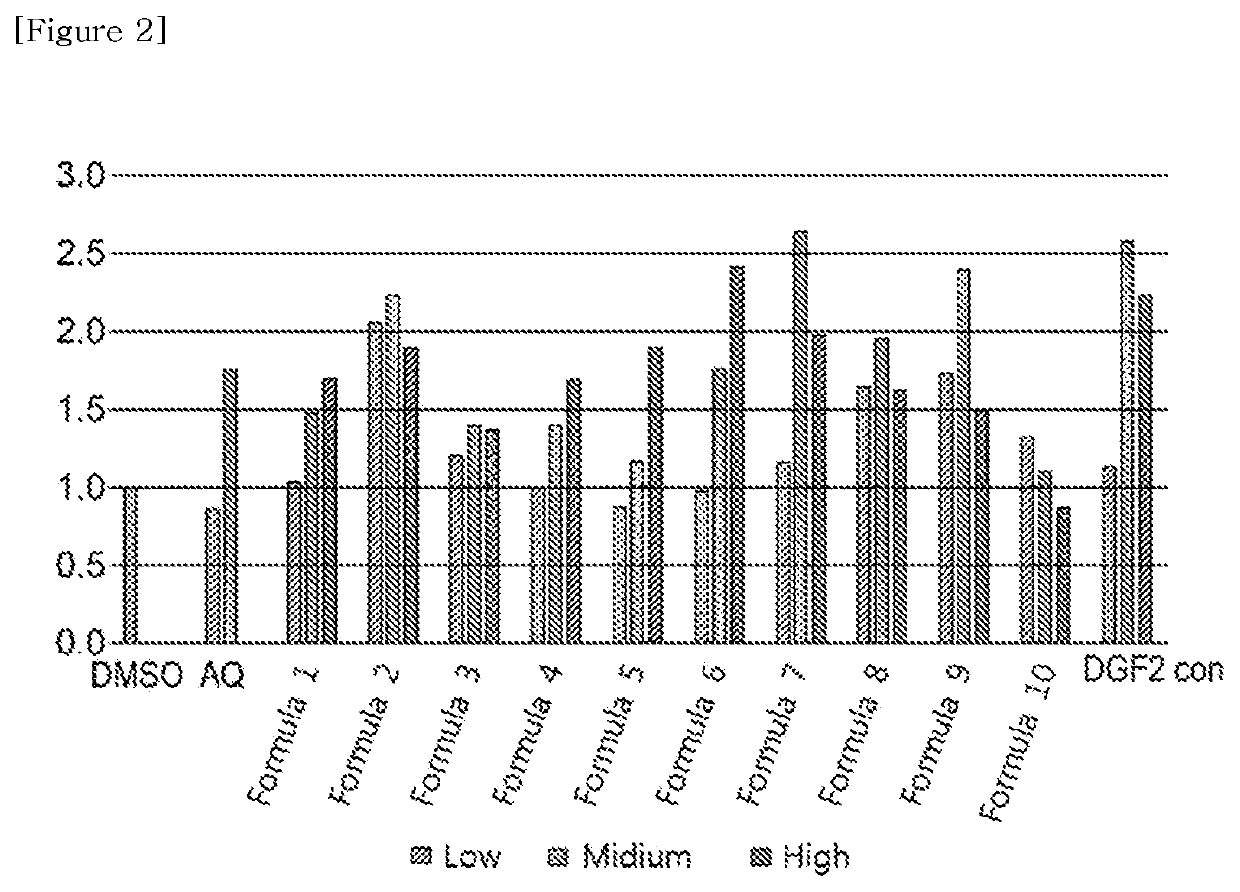
[0157]As in Example 2, Nurr1 activity according to the concentration of the diterpene isolated from the extract of Daphne genkwa was confirmed through luciferase analysis.
[0158]Specifically, after synthesizing a vector in which the gene having the nucleotide sequence (5′-CTCGGAGGACAGTACTCCG-3′, SEQ ID NO:1) to which the GLA4 gene can bind is repeated 8 times to the reporter gene, luciferase, is synthesized, 3 types of plasmid DNA, including DNA containing Nurr1-LBD and DNA with β-galactosidase, were transfected into BE(2)C cells. After 6 hours, the compounds 1 to 10 separated in Example 2 were treated according to the concentrations in Table 1 below. The cells thus treated were cultured in a 5% carbon dioxide incubator at 37° C. for 20 hours, and then luciferase analysis was performed. As a control, 0.1% DMSO was used, and at this time, the activity was increased by multiple, and Amodiaquine was used as a positive control.
TABLE 1Final treatment concentrati...
example 4
n Activity on Nitric Oxide Production in a Microglia BV-2 Cell
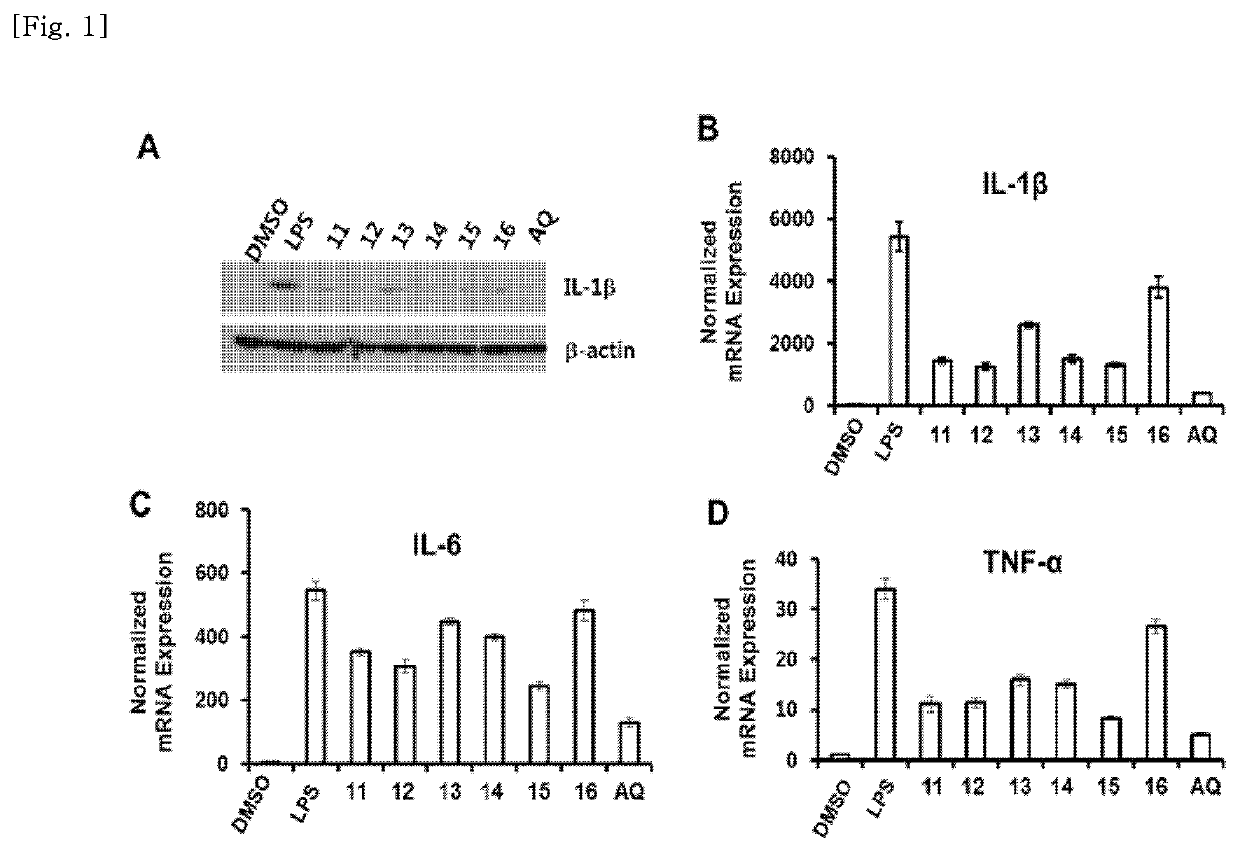
[0161]The death of cranial nerve cells due to an inflammatory reaction in a microglia cell has been reported as one of the main causes of degenerative brain diseases such as dementia and Parkinson's disease. (Sarkar S et al., Neurotoxicology, 44, 250-262 (2014); Bower J H et al., Neurology, 67, 494-496 (2006)). Accordingly, the inhibition activity on nitric oxide production, a representative inflammatory factor, was investigated for the compounds isolated from Example 2 in a microglia. Specifically, microglia BV-2 cells were put into a 96-well plate at 5×104 cells / well, and cultured for 2 days, followed by incubation with LPS (1 mg / mL) for 24 hours with the compound isolated in Example 2 above. The culture supernatant was measured for absorbance at 540 nm using the Griess reagent to quantify nitrite to investigate the amount of nitric oxide production. Minocyline was used as a positive control.
[0162]As a result of the inv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com