Method for synthesis of N-homocysteine thiolactonyl retinamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of N-Homocysteine Thiolactonyl Retinamide
The compound produced by methods described in this invention, N-homocysteine thiolactonyl retinamide, is also abbreviated as thioretinamide. This compound is an N-substituted derivative of homocysteine thiolactone which is conjugated to retinoic acid or retinamide. Thioretinamide has antineoplastic, chemopreventive, and antiatherogenic properties. Synthesis of this compound by organic methods allows the investigation of the mechanisms of action of these properties. The steps involved in the synthesis of thioretinamide are as follows:
Synthesis of N-homocysteine thiolactonyl retinamide (thioretinamide) can also be carried out by alternative methods as disclosed herein. Under conditions of reduced light and an atmosphere of argon, all-trans-retinoic acid, 0.666 M (0.200 grams) (Sigma Chemical Co., Missouri), was dissolved in 20 ml of argon protected anhydrous tetrahydrofuran (THF). After stirring ten minutes, the transparent yellow sol...
example 2
Modifications for the Synthesis of Thioretinamide
All of the compositions and / or methods disclosed and claimed herein can be made and executed without undue experimentation in light of the present disclosure. While the compositions and methods of this invention have been described in terms of preferred embodiments in Example 1, it will be apparent to those of skill in the art that variations may be applied to the compositions and / or methods disclosed and in the steps or in the sequence of steps of the method described in Example 1 without departing from the concept, spirit and scope of the invention. All such similar substitutes and modifications apparent to those skilled in the art are deemed to be within the spirit, scope and concept of the invention are presented in this Example. For example, the factors can be changed in the procedure described in Example 1, without deviating from the scope of the invention.
Coupling Reagents
Different coupling agents, other than those described in...
example 3
Analysis of N-Homocysteine Thiolactonyl Retinamide
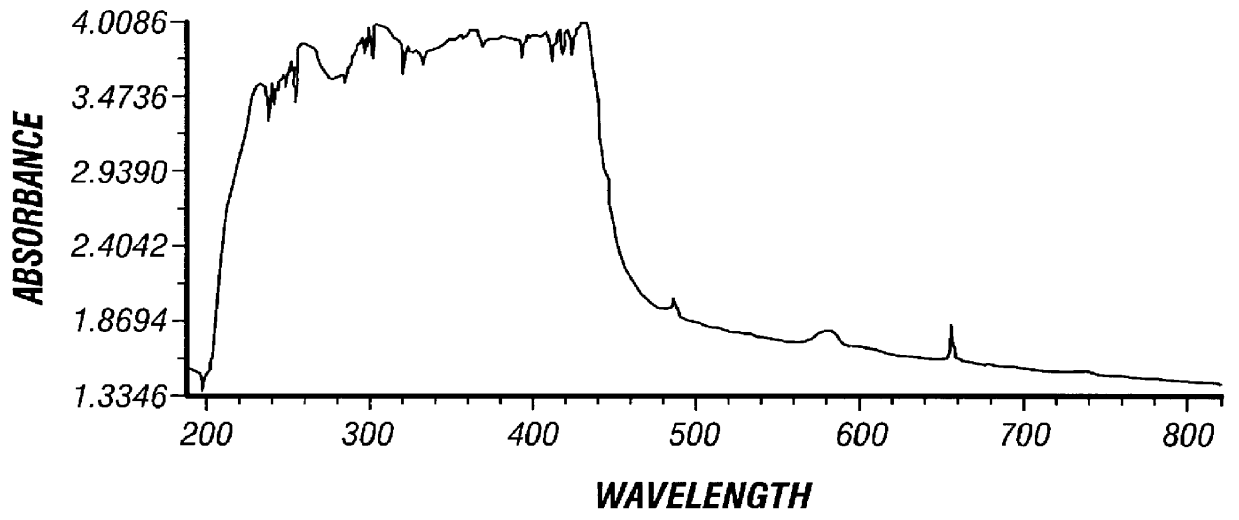
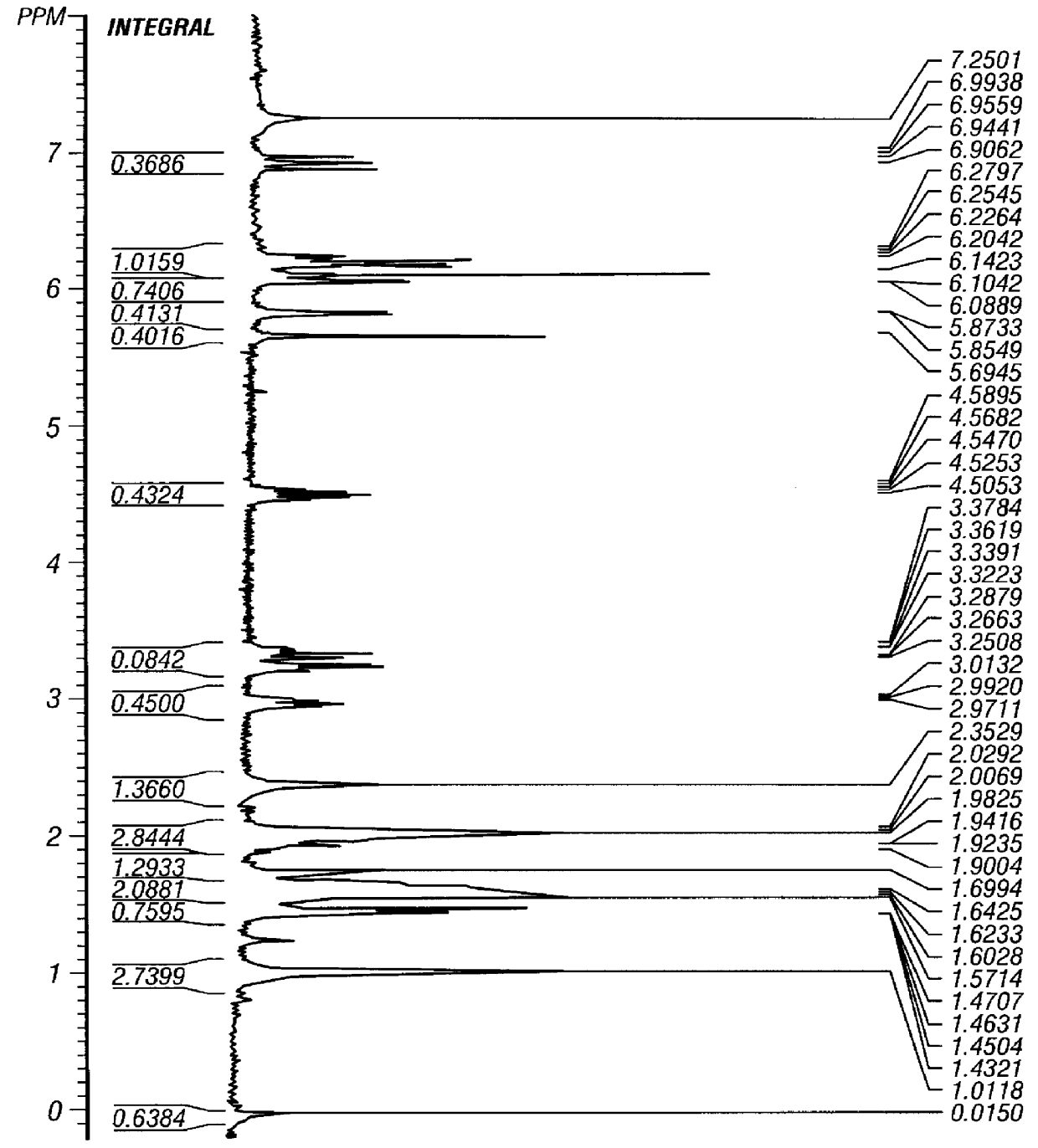
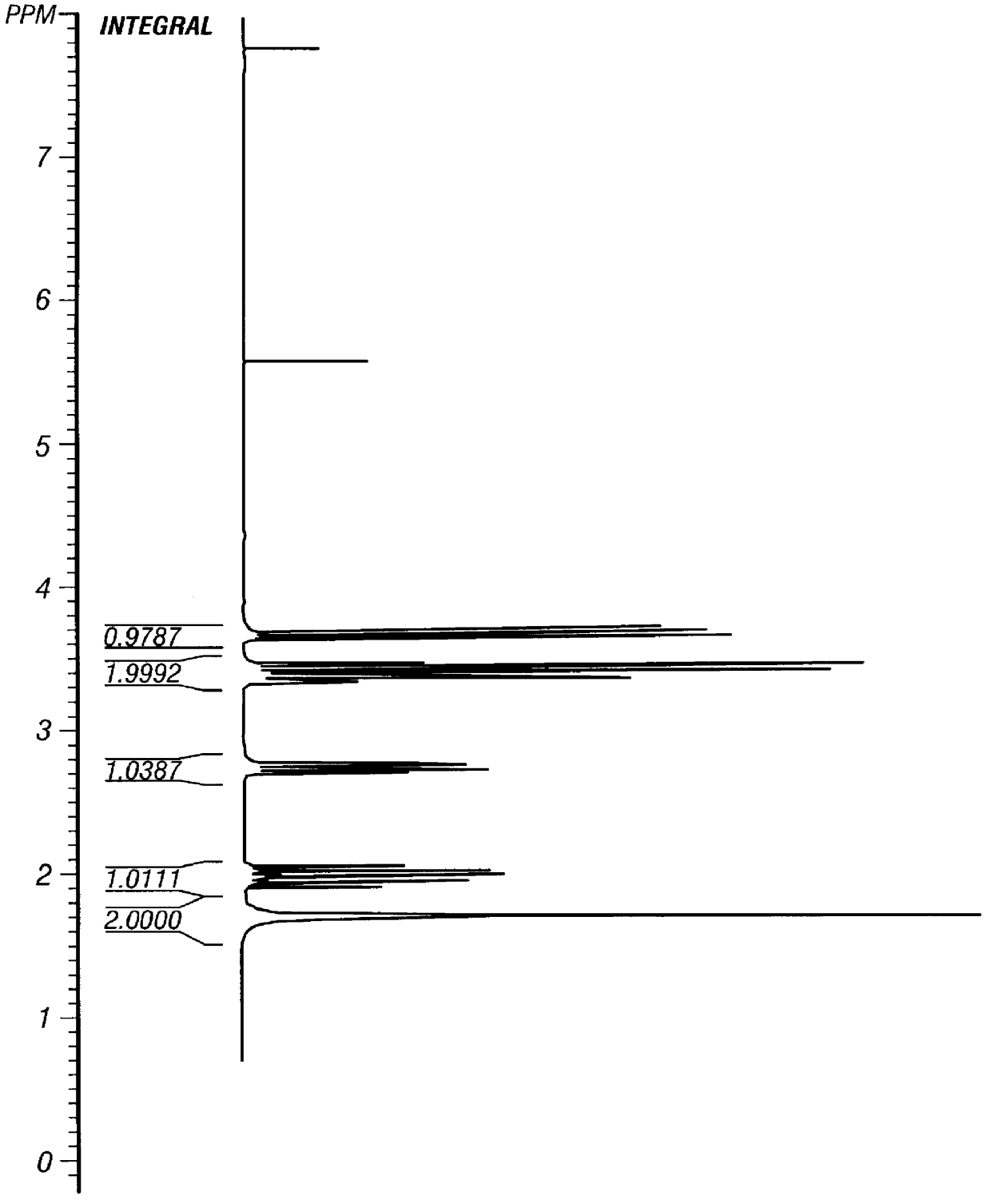
Verification of thioretinamide synthesis was performed by using proton and carbon N.M.R. and mass spectroscopy. The proton N.M.R. of thioretinamide (FIG. 2) shows both homocysteine thiolactone (FIG. 3) and retinoic acid (FIG. 4) as constituents of thioretinamide. The proton N.M.R. of thioretinamide (FIG. 2) at 1.0-2.4 ppm consists of singlet peaks integrating for the three methyl group hydrogens of the retinoic acid component of thioretinamide. Peaks at 2.9-4.6 ppm correspond to the protons of the homocysteine thiolactone ring of thioretinamide. The peaks at 5.7-7.0 ppm represent the unsaturated protons, vinyl protons, of the retinoic acid component of thioretinamide. The conjugation of homocysteine thiolactone and retinoic acid at the homocysteine thiolaconyl amide is indicated by downfield shifting of proton signals of the amide hydrogen thioretinamide N.M.R. The synthesis of thioretinamide reported by McCully and Vezeridis (1987a)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com