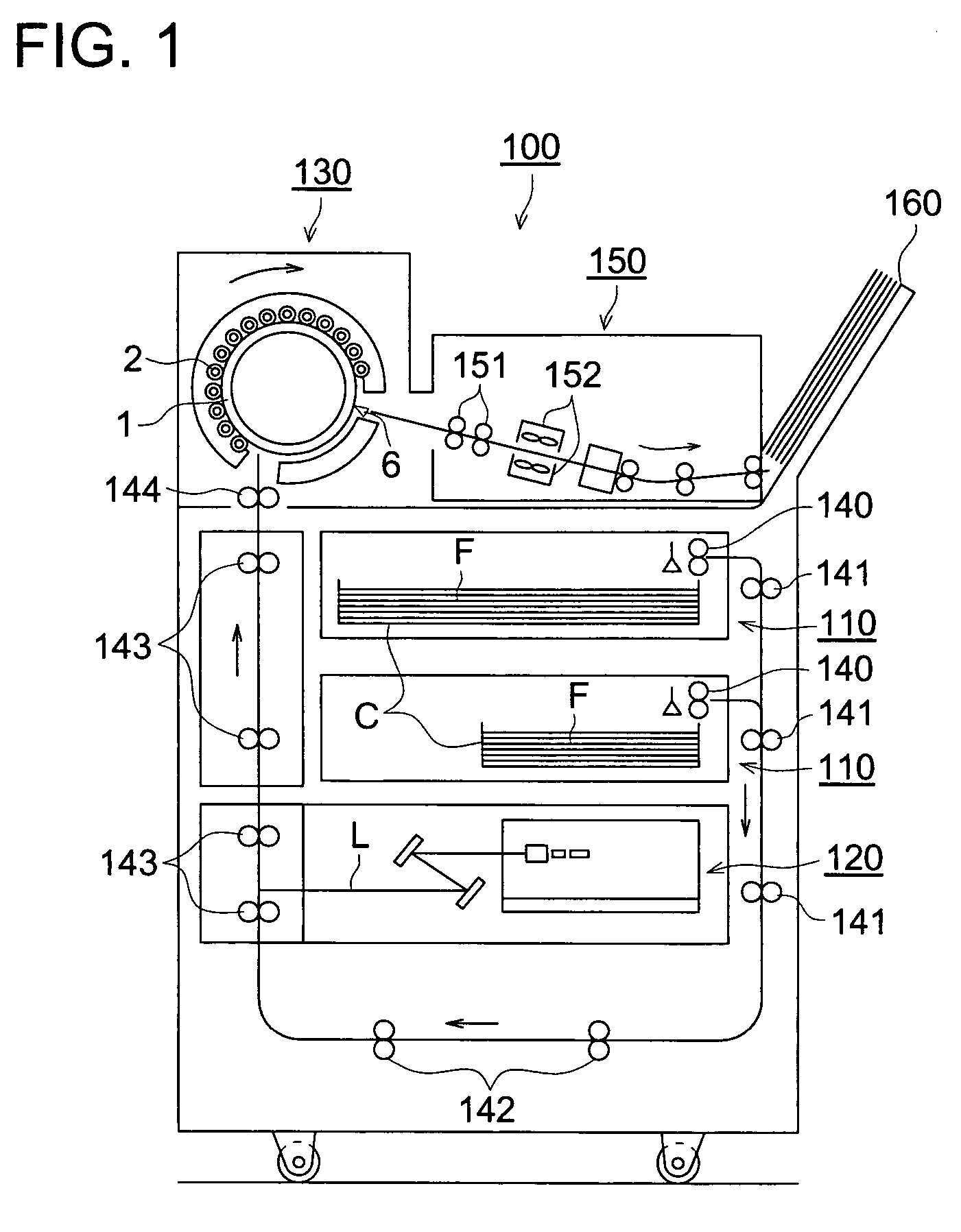
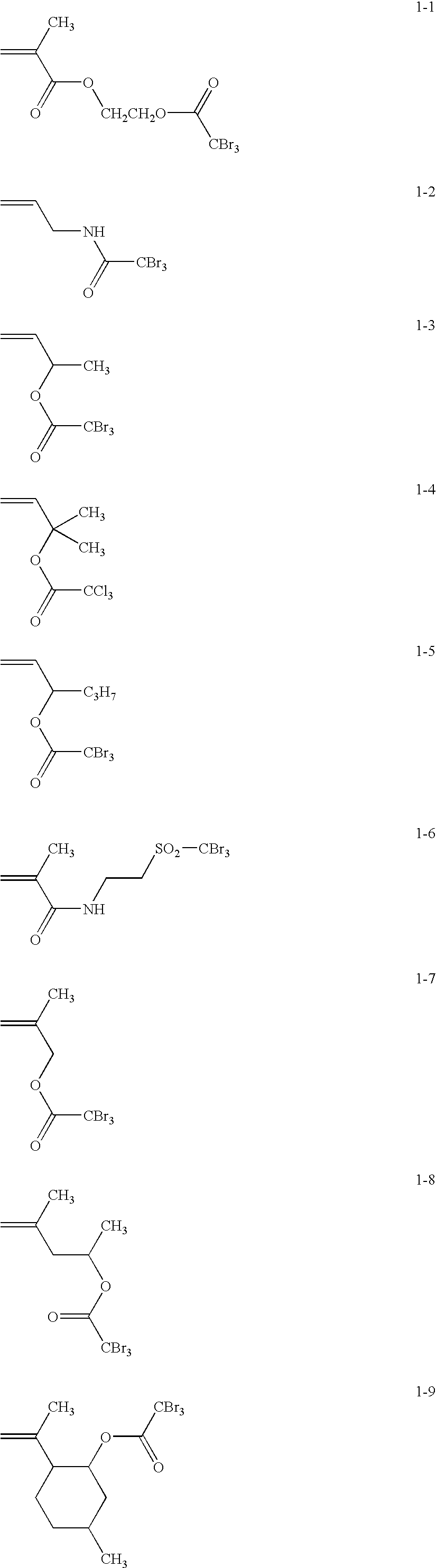
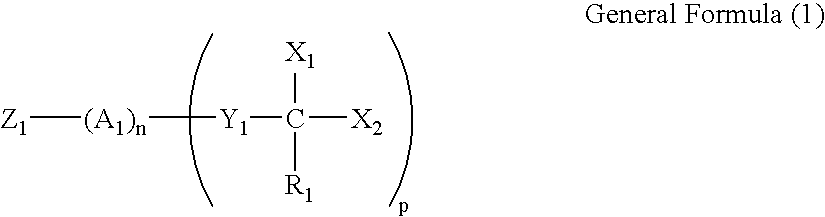Silver salt photothermographic dry imaging material and production method of the same
a technology of photothermographic and dry imaging material, which is applied in the field of silver salt photothermographic dry imaging material, can solve the problems of difficult mixing of above grains with polymers dissolved in organic solvents or organic silver particles dispersed in organic solvents, and difficulty in dispersing, so as to achieve high photographic speed, improve silver color tone, and retard fog formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthetic example 1
Synthesis of Exemplified Compound 1-1
[0194]Mixed successively were 5.8 g of triethanolamine, 25 ml of dichloromethane, and 5.0 g of 2-hydroxyethyl methacrylate. While cooled with iced water, a solution prepared by dissolving 12.1 g of tribromoacetyl chloride in 10 ml of dichloroethane, was dripped into the resulting mixture. After dripping, the resultant mixture was stirred at room temperature for three hours, and then 100 ml of ethyl acetate was added. Thereafter, the resultant organic layer was washed successively with 50 ml of 1 mol / L hydrochloric acid, 50 ml of a saturated sodium hydrogencarbonate solution, and 50 ml of a saturated sodium chloride solution. Dehydration was performed employing magnesium sulfate, and after filtration, concentration under reduced pressure was carried out, whereby crude crystals were obtained. The resultant crystals were recrystallized employing ethanol, whereby targeted Exemplified Compound 1-1 (11.0 g) was obtained.
synthetic example 2
Synthesis of Exemplified Compound 2-2
[0195]Mixed successively were 6.3 g of triethanolamine, 25 ml of dichloromethane, and 5.0 g of 4-vinylphenol. While cooled with iced water, a solution prepared by dissolving 14.4 g of tribromoacetyl chloride in 10 ml of dichloroethane, was dripped into the resulting mixture. After dripping, the resultant mixture was stirred at room temperature for three hours, and then 100 ml of ethyl acetate was added. Thereafter, the resultant organic layer was washed successively with 50 ml of 1 mol / L hydrochloric acid, 50 ml of a saturated sodium hydrogencarbonate solution, and 50 ml of a saturated sodium chloride solution. Dehydration was performed employing magnesium sulfate, and after filtration, concentration under reduced pressure was carried out, whereby crude crystals were obtained. The resultant crystals were recrystallized employing ethanol, whereby targeted Exemplified Compound 2-2 (13.2 g) was obtained.
synthetic example 3
Synthesis of Homopolymer Having a Repeated Unit of Exemplified Compound 1-1
[0196]Mixed successively were 10 g of above Exemplified Compound 1-1, 80 ml of dehydrated tetrahydrofuran, and 0.3 g of boron trifluoride-diethyl ether complex, and the resultant mixture was refluxed for ten hours while heated. After cooling, concentration under reduced pressure was performed. The resultant residues were dissolved in tetrahydrofuran and purified employing repeated precipitation employing methanol, whereby 5 g of a homopolymer of a number average molecular weight of 5,000 was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| isoelectric point | aaaaa | aaaaa |
| grain diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com