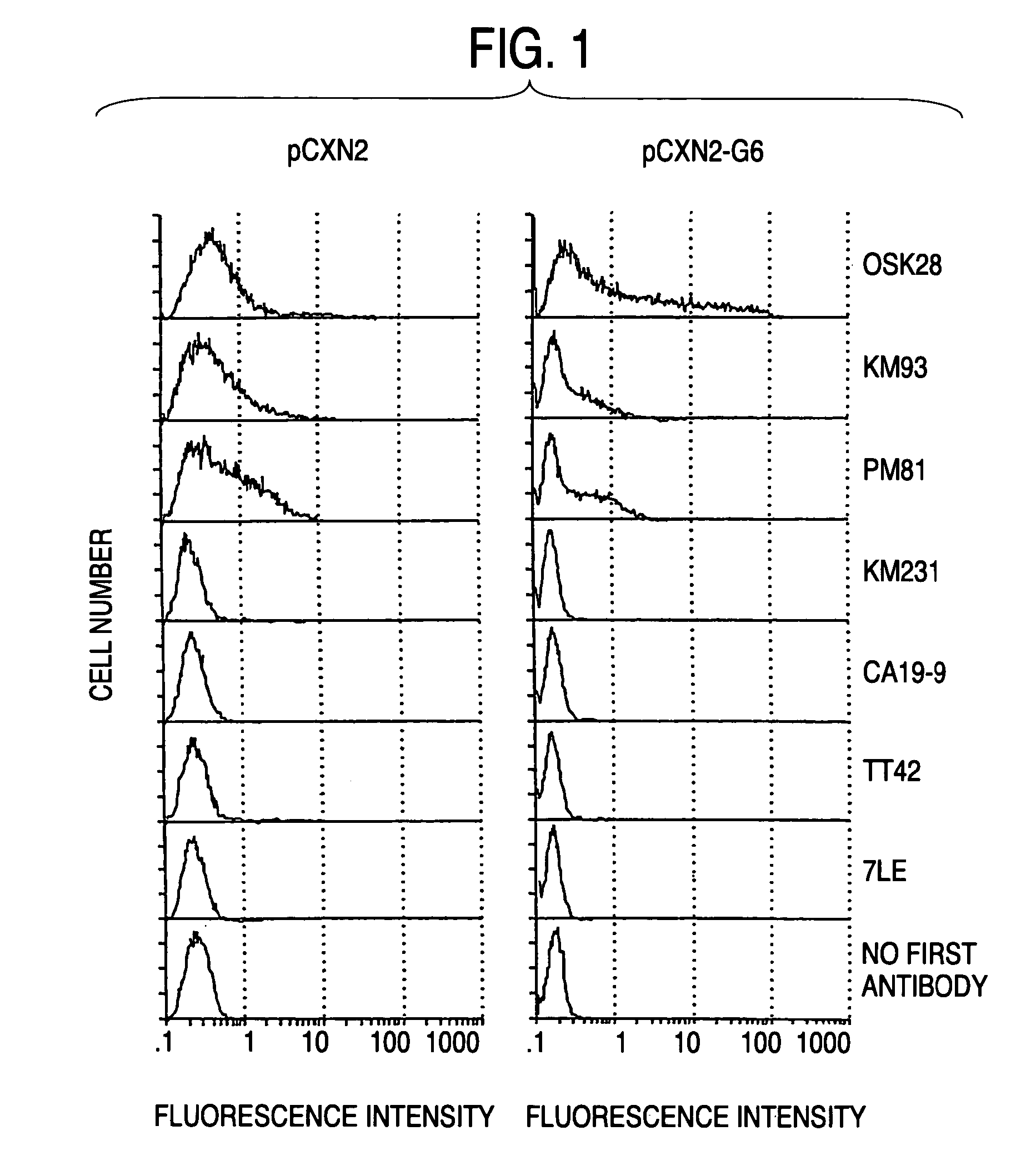
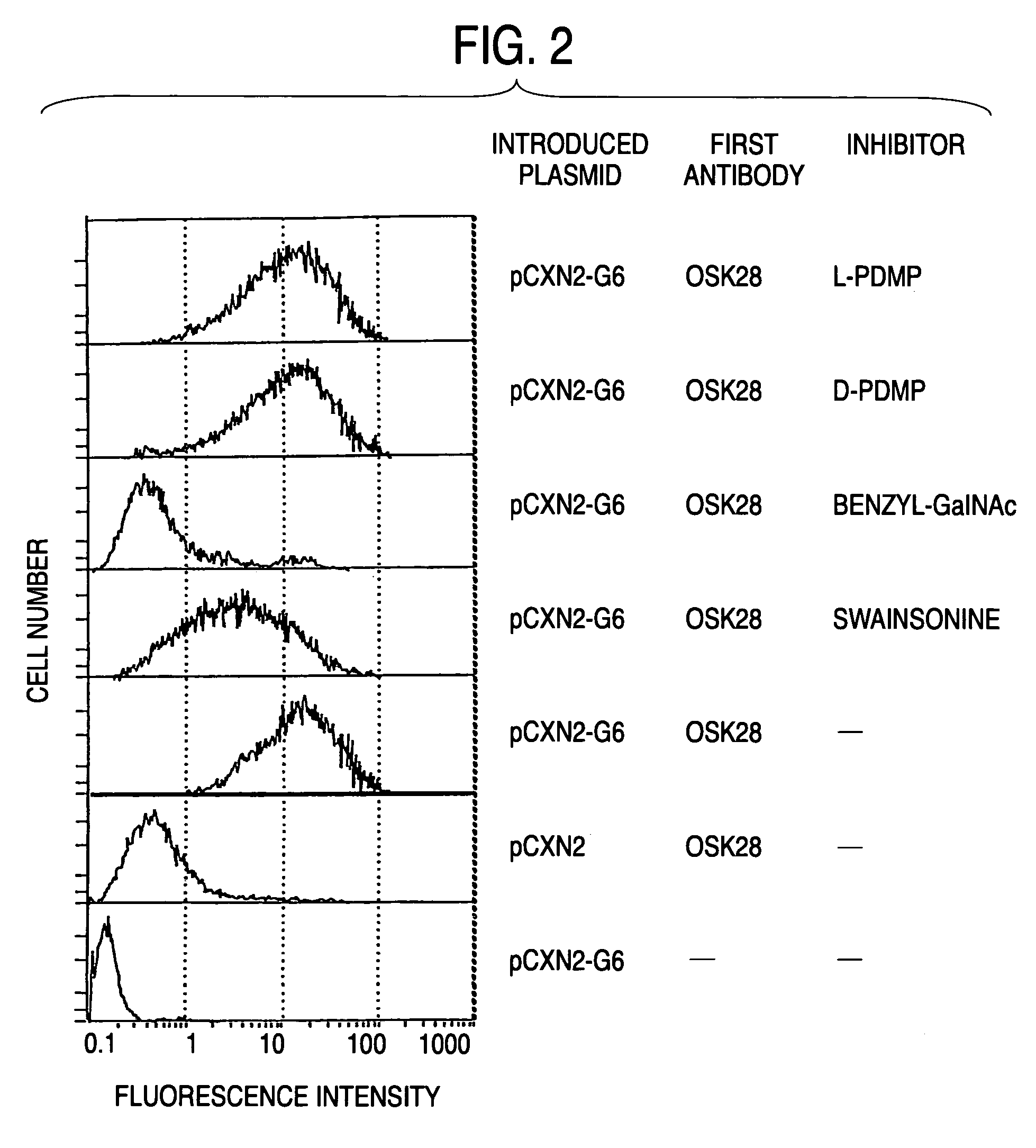
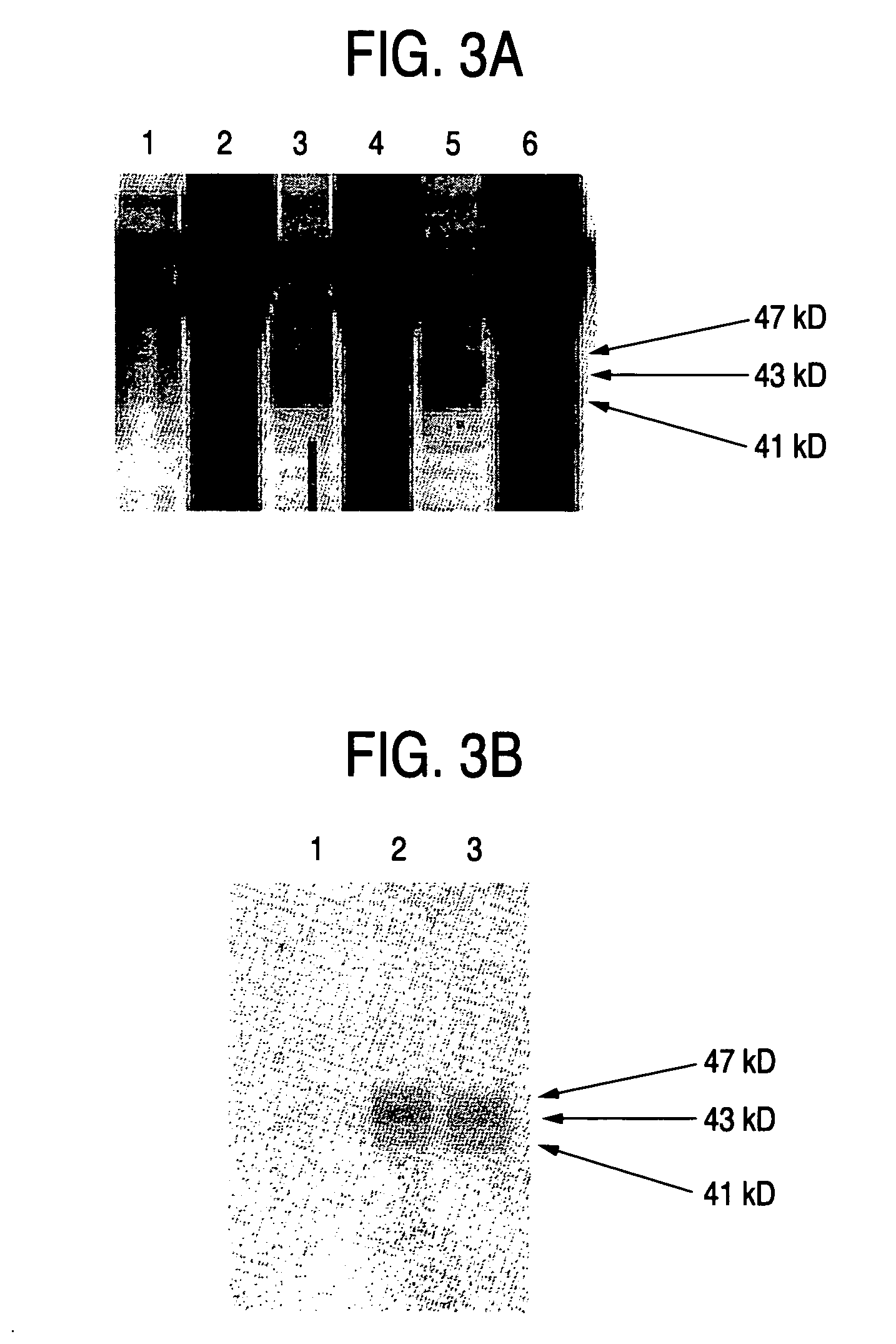
Process for producing sugar chains using beta1,3-N-acetylglucosaminyltransferase
a technology of acetylglucosaminyltransferase and sugar chain, which is applied in the field of new polypeptides, can solve the problems of difficult to synthesize a large amount of poly-n-acetylglucosamine sugar chains, no reports to date on the efficient production of recombinant glycoproteins to which poly, and achieve the effect of efficiently culture the transforman
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of β1,3-galactosyltransferase Homologue (Rat G6) Gene from a Rat Tibia-derived cDNA Library
(1) Preparation of RNA
[0432]From rat tibia, 2.2 mg of total RNA was prepared by the guanidine thiocyanate-cesium trifluoroacetate method [Methods in Enzymology, 154, 3 (1987)]. Next, 15.7 μg of mRNA was obtained as poly(A)+ RNA by passing 2.0 mg of the total RNA through an oligo(dT) cellulose column (manufactured by Collaborative Research).
(2) Preparation of cDNA Library
[0433]Using 4.0 μg of the mRNA obtained in the above (1), synthesis of cDNA, ligation of BamHI adapter and digestion with NotI were carried out according to the linker primer method [Preparation Methods of Gene Library (Idenshi Library no Sakusei-ho), edited by Hirosho Nojima, Yodo-sha, 1994]. A cDNA library in which 5′-terminal of cDNA is always present in the BamHI site side of the vector was constructed by inserting the thus obtained double-stranded cDNA between BamHI site and NotI site of a plasmid pBluescript II SK...
example 2
Search of Gene Encoding Human G6
[0436]Human G6 gene corresponding to the rat G6 cDNA obtained in Example 1 was searched. As a result of the search of genes having homology with the nucleotide sequence (represented by SEQ ID NO:3) of the cDNA contained in OVX2-038 obtained in Example 1, or genes having a possibility to encode polypeptides having homology with the polypeptide (represented by SEQ ID NO:32) considered to be encoded by the cDNA at amino acid level from gene data bases by using the programs of BLAST [J. Mol. Biol., 215, 403-410 (1990)] and FrameSearch [manufactured by Compugen], one EST (expressed sequence tag) sequence (GenBank No. AI039637) was found. Since the EST sequence is 428 bp, a total amino acid sequence of a polypeptide encoded by a gene corresponding to this EST and the function of the polypeptide cannot be found from this sequence information alone.
example 3
Cloning of Human G6 cDNA
[0437]Cloning of candidate gene fragments was attempted by designing a primer set specific for the EST sequence (GenBank No. AI03937) found in Example 2. As the primers, CB-462 having the nucleotide sequence represented by SEQ ID NO:4 and CB-464 having the nucleotide sequence represented by SEQ ID NO:5 were used. Using the primer set, the presence of human G6 cDNA was examined by PCR by using single-stranded cDNAs prepared from various organs, or various cDNA libraries, as templates. As a result, a DNA fragment of about 250 bp was amplified when a cDNA library derived from a human colon cancer cell line Colo205 or a cDNA library derived from human gastric mucosa was used as a template.
[0438]A human G6 cDNA clone of about 3 kb was obtained by screening the above two cDNA libraries by using the amplified fragment as a probe. Since the clone was lacking the 5′-terminal portion, a 5′-terminal side DNA of the human G6 cDNA was obtained by using 5′ RACE method. Ful...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com