Corrosion-responsive coating formulations for protection of metal surfaces
a technology for corrosion-responsive coatings and metal surfaces, applied in the direction of non-metal conductors, other chemical processes, conductors, etc., can solve the problems of affecting the use of hexavalent chromium in the aerospace sector, affecting the cost of direct loss of metallic corrosion, and lacking current coating strategies, etc., to achieve the effect of low spontaneous release ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
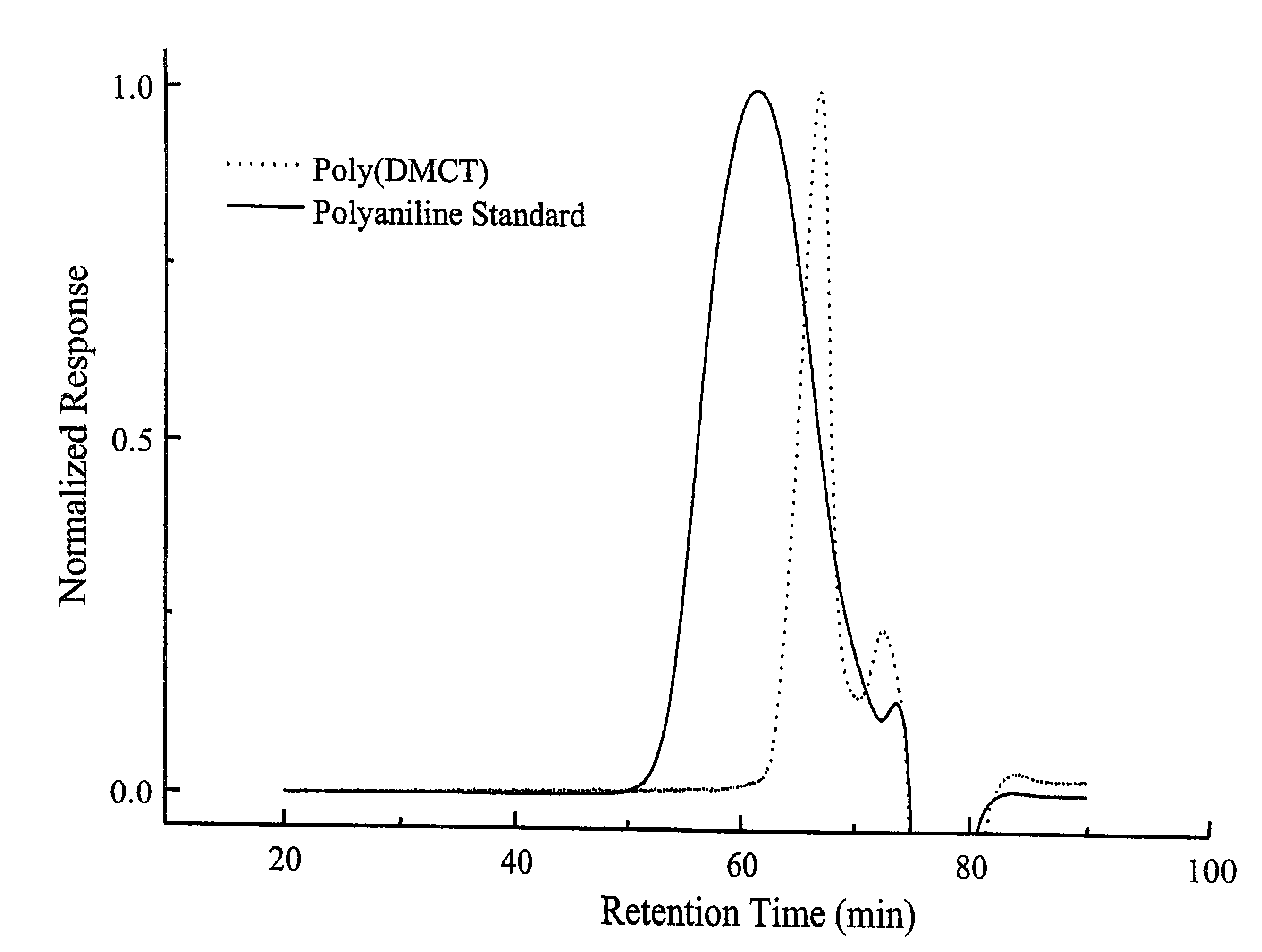
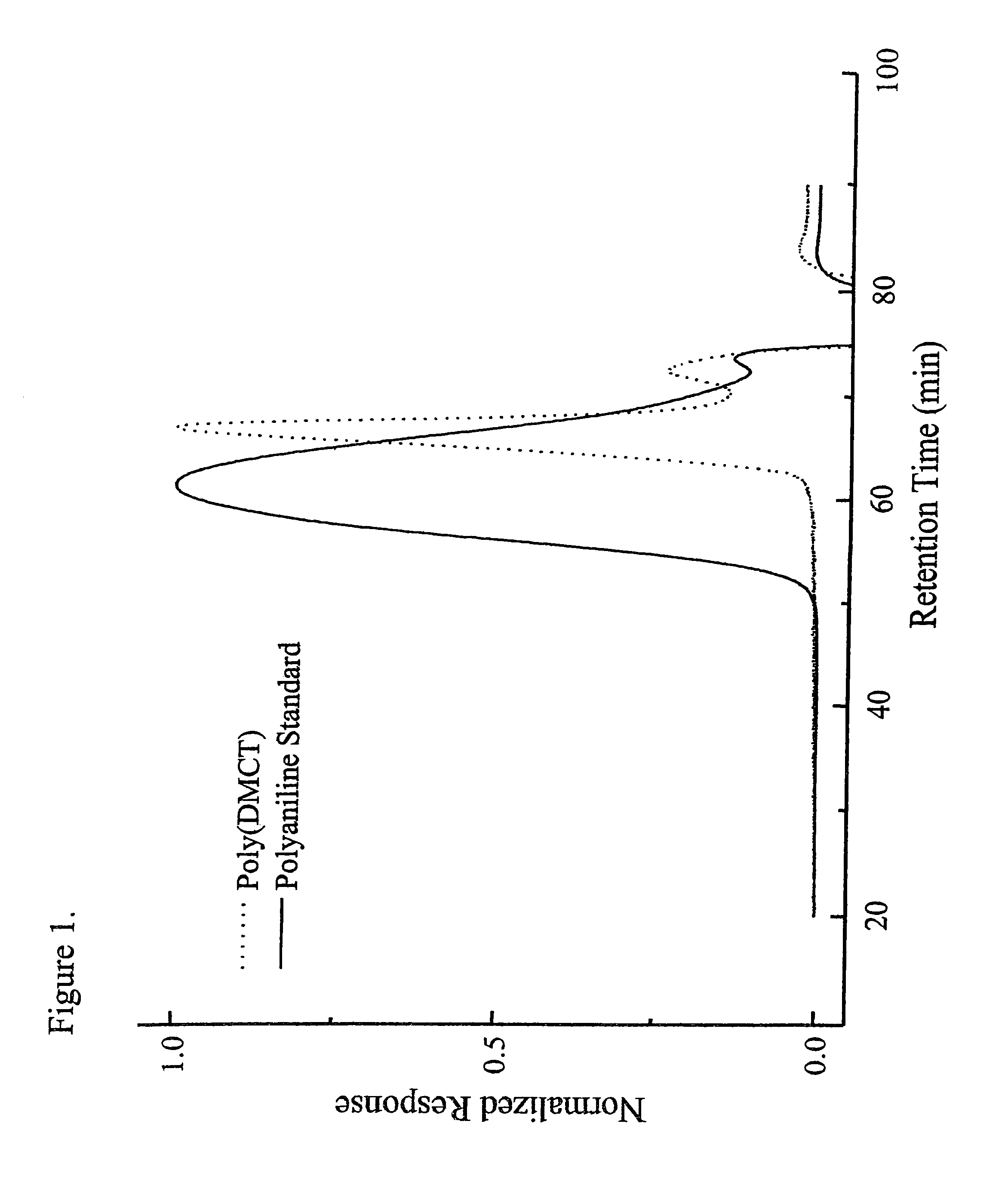
[0102]This illustrates the production of poly(2,5-dimercapto-1,3,4-thiadiazole).
[0103]2,5-dimercapto-1,3,4-thiadiazole (25 grams, DMcT, available from Sigma-Aldrich, Milwaukee, Wis.) was added to 50 / 50 deionized water / methanol (1500 ml). Sodium hydroxide (6.66 grams) was then added to the mixture with stirring until the mixture became a clear transparent yellow. The mixture was heated to about 45° C. with stirring. In a separate flask, iodine (42.13 grams) was dissolved in methanol (400 ml) transferred to an addition funnel that is attached to the round-bottom flask holding the DMcT mixture. The iodine solution was added dropwise to the DMcT mixture in the flask with stirring over a period of about 30 minutes. A precipitate formed immediately and was initially white, but became reddish brown as the iodine solution was added. After stirring for 2 hours, the product was recovered by filtration, and the product was washed with acetonitrile, methanol and deionized water. The solid produ...
example 2
[0104]This illustrates the production of polyaniline doped with (2,5-dimercapto-1,3,4-thiadiazole).
Synthesis of DMcT-salt of polyaniline (Blender Method):
[0105]2,5-dimercapto-1,3,4-thiadiazole (93 grams) was ground in a mortar with a pestle to a fine powder. The powder was added to deionized water in a Waring blender and emulsified in the blender for 1 minute. Aniline (57 grams) was added to the mixture in the blender and emulsified for 1 minute. The mixture in the blender was transferred to a 3 liter round-bottom jacketed flask that was cooled to about 5° C. and blanketed with nitrogen. Ammonium peroxidisulfate (170 grams, APDS) was dissolved in deionized water and transferred to an addition funnel, which was attached to the round-bottom flask. The APDS solution was then added dropwise to the mixture in the flask over a period of about 15 minutes while maintaining the temperature of the mixture in the flask below about 5° C. The mixture was stirred for 3 hours at about 5° C. under ...
example 3
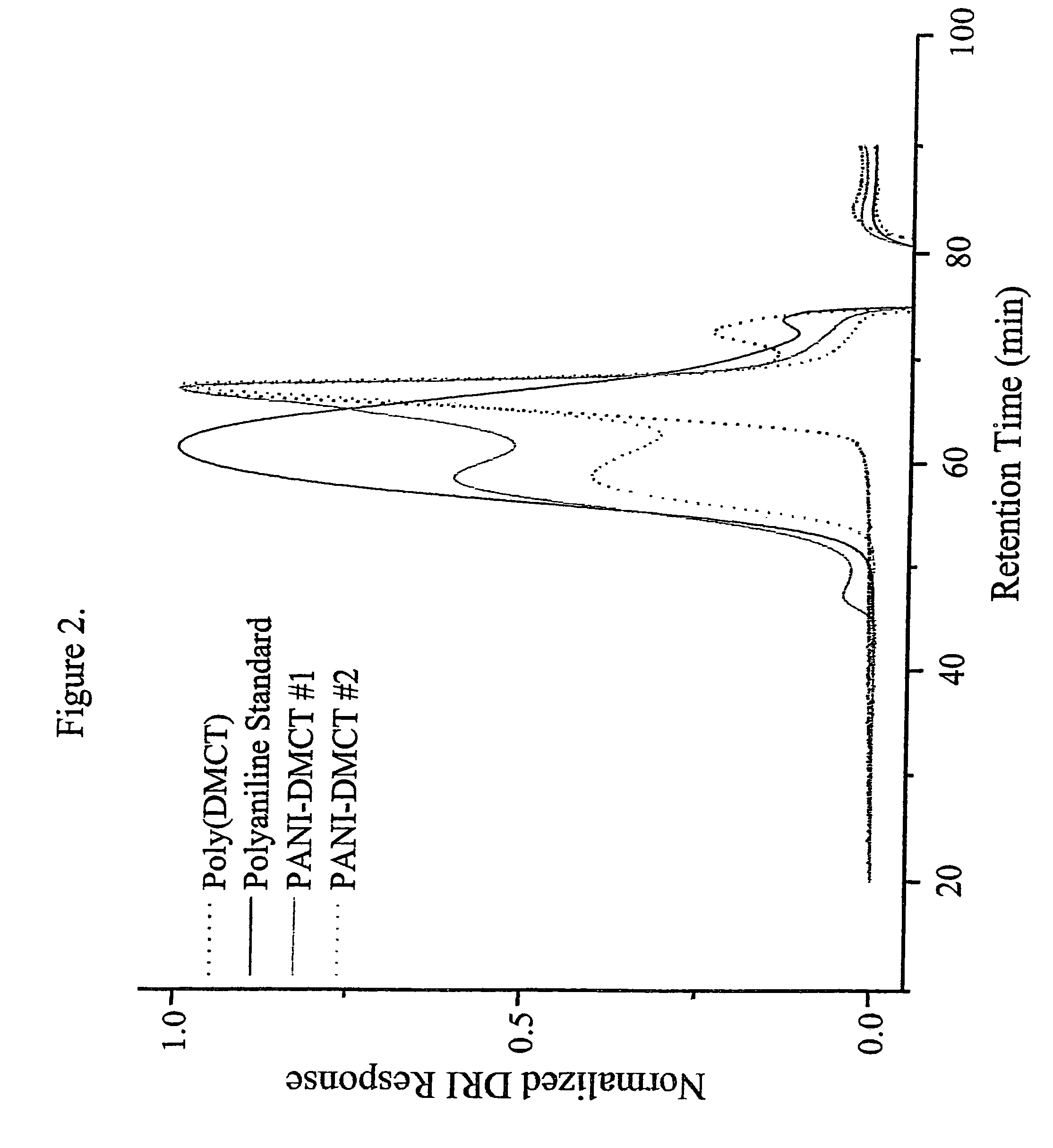
[0114]This illustrates the production of UV-curable coating formulations containing (2,5-dimercapto-1,3,4-thiadiazole), poly(2,5-dimercapto-1,3,4-thiadiazole), and polyaniline doped with (2,5-dimercapto-1,3,4-thiadiazole).
[0115]A measured amount of a UV-curable resin (160 g; available as FD3007CI UV, from Allied PhotoChemical, Kimball, Mich.) was charged to an Eiger Mini Mill (Model 100 VSE; Eiger Machinery, Inc., Grayslake, Ill.), and an amount (17.78 g dry weight) of a corrosion-responsive agent, selected from 2,5-dimercapto-1,3,4-thiadiazole (DMcT), poly(2,5-dimercapto-1,3,4-thiadiazole), and polyaniline doped with (2,5-dimercapto-1,3,4-thiadiazole) (Pani-DMcT) was added as a solid material to the liquid to give a mixture that was 10% by weight corrosion-responsive agent. The solids and the liquid were milled until the solids were of the desired particle size and were well-dispersed in the liquid.
[0116]A known weight (100 g) of the 10% w / w mixture was drawn from the mill, and 77....
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com