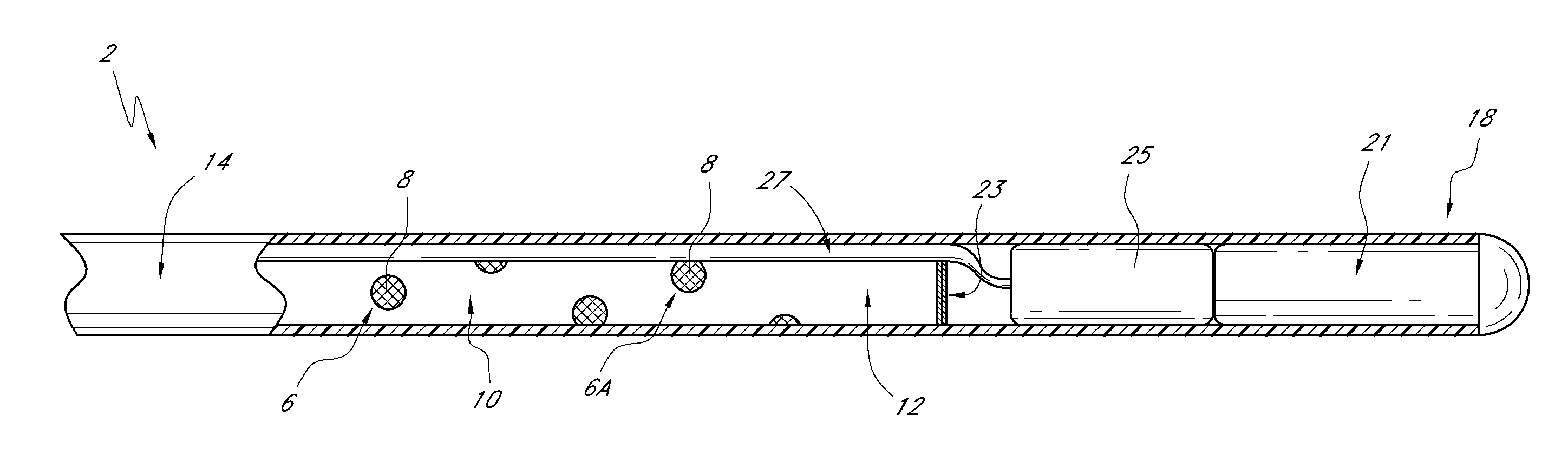
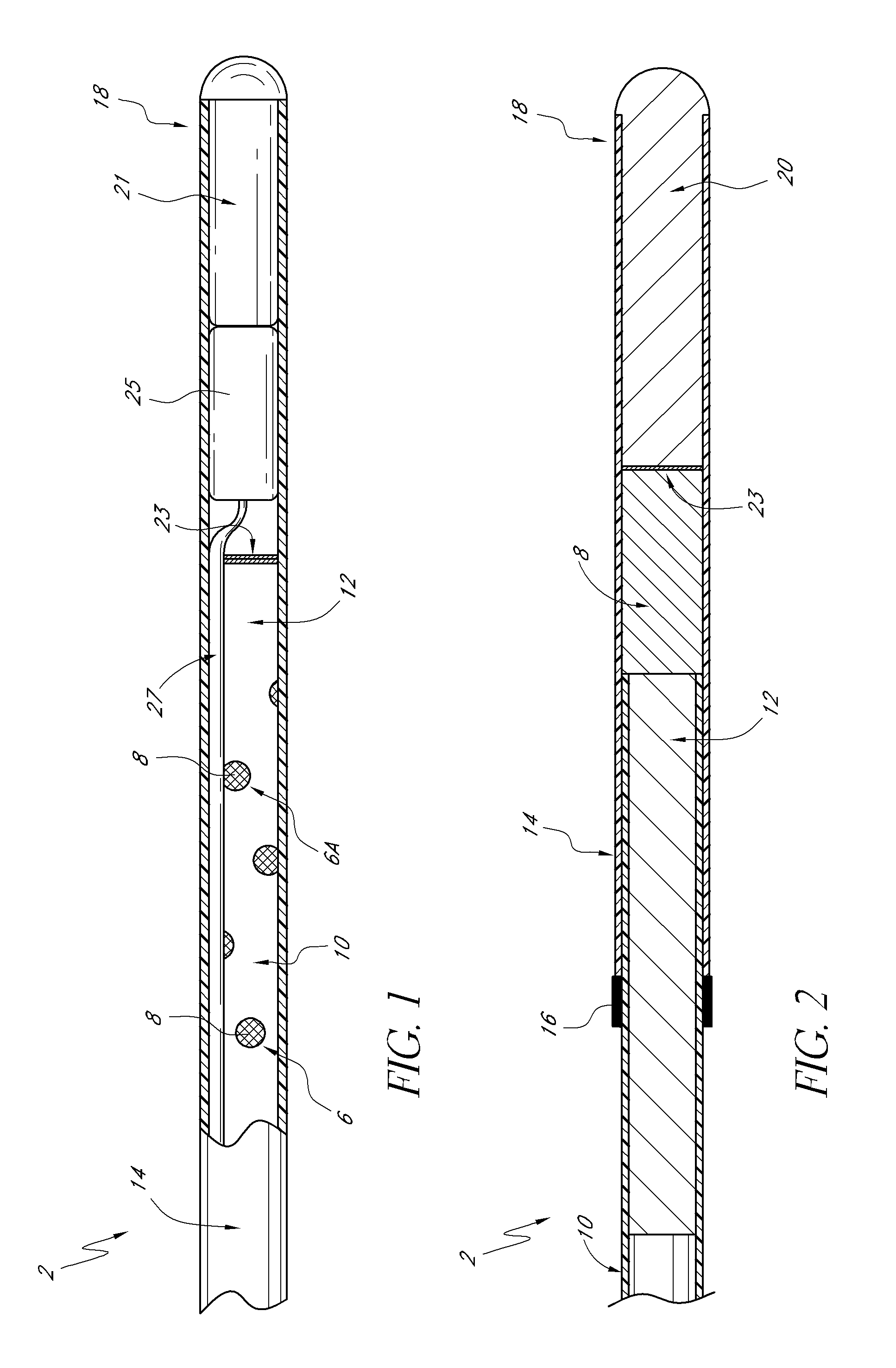
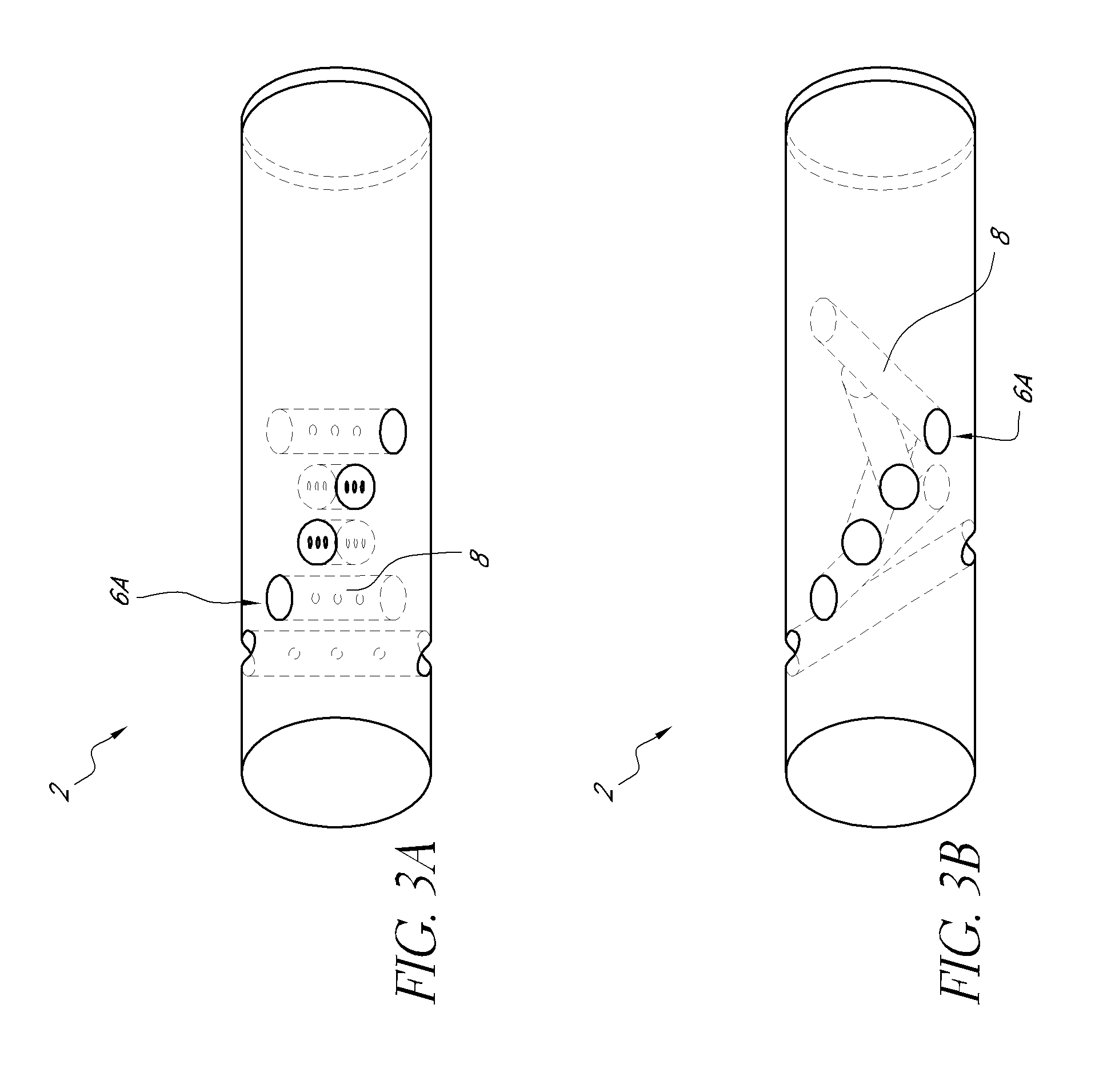
Sensors with thromboresistant coating
a technology of thrombosis and sensors, applied in the direction of instruments, catheters, analysis using chemical indicators, etc., can solve the problems of blood clots or thromboses around the sensor, severe health problems, and impair the functionality of the sensor and/or the health of the patient, so as to reduce the thrombosis of the analyte sensor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
Example 1
Application of Thromboresistant Coating
[0140]An optical glucose sensor as described above (see e.g., FIGS. 1-4) was prepared for coating with benzalkonium / heparin by immersing the portion of the sensor to be coated in a pH 3 phosphate buffered saline solution (although it is feasible to use many types of aqueous buffer solutions or even just water).
[0141]A coating solution of 1.5% (by weight) benzalkonium heparin in isopropanol (distributed by Celsus Laboratories, Inc. 12150 Best Place, Cincinnati Ohio 45241 as Benzalkonium heparin solution in isopropyl alcohol, 887 U / mL, Product Number BY-3189) was added to a test tube. After equilibrating in the buffered saline solution, the distal end portion of the sensing end of the sensor was immersed in the benzalkonium heparin solution and immediately removed (with the time of immersion in the benzalkonium heparin solution being approximately one second). The wet sensor was allowed to air dry for approximately 1 minute, resulting in...
example 2
Preparation of Sensor Blank
[0143]A sensor blank was prepared from a polyethylene microporous membrane (of 0.017 inch outside diameter) surrounding a poly(methyl methacrylate) optical fiber (of 0.010 inch diameter). The polyethylene microporous membrane was obtained from Biogeneral 9925 Mesa Rim Road, San Diego Calif. 92121-2911). The distal end of the sensor blank (the end to be coated) is heat welded to a rounded polyethylene plug. The other end is sealed with a silicone backfill. The distal end was then immersed in the buffered saline solution of Example 1 for about 18 hours (although a shorter time interval would also have been suitable). Finally, the distal end of the sensor blank was immersed in the coating solution of Example 1 and subsequently air dried as in Example 1. The steps of immersing in the coating solution and air drying were repeated four times.
example 3
Comparison of Coated Sensor and Coated Sensor Blank
[0144]Coated sensors and coated sensor blanks, prepared as described in Examples 1 and 2, each having five dip coats of heparin / benzalkonium applied, were subjected to handling tests as follows.
[0145]Sensors consisted of a 1.3-inch long hollow, microporous HDPE membrane (0.017 inches O.D., Biogeneral 9925 Mesa Rim Road, San Diego Calif. 92121-2911, this is a custom part) butt-welded to a 1.0-inch long, smooth (nonporous) HDPE tube. The microporous end was heat-welded to a rounded polyethylene plug. Inside of the hollow assembly was threaded a 0.010 inch PMMA optical fiber The smooth HDPE end was filled with silicone backfill up to, but not including, the microporous membrane. The area between the PMMA optical fiber and the hollow microporous membrane was filled with a dimethyl acrylamide gel which also contained covalently-bound fluorescent dye and quencher. The sensor was prepared for application of the coating comprising heparin a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com