Human neutrophil collagenase splice variant
a neutrophil and collagenase technology, applied in the field of human neutrophil collagenase splice variants, to achieve the effect of preventing the expression of human mmp-8alt and reducing or preventing the effect of human mmp-8alt polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
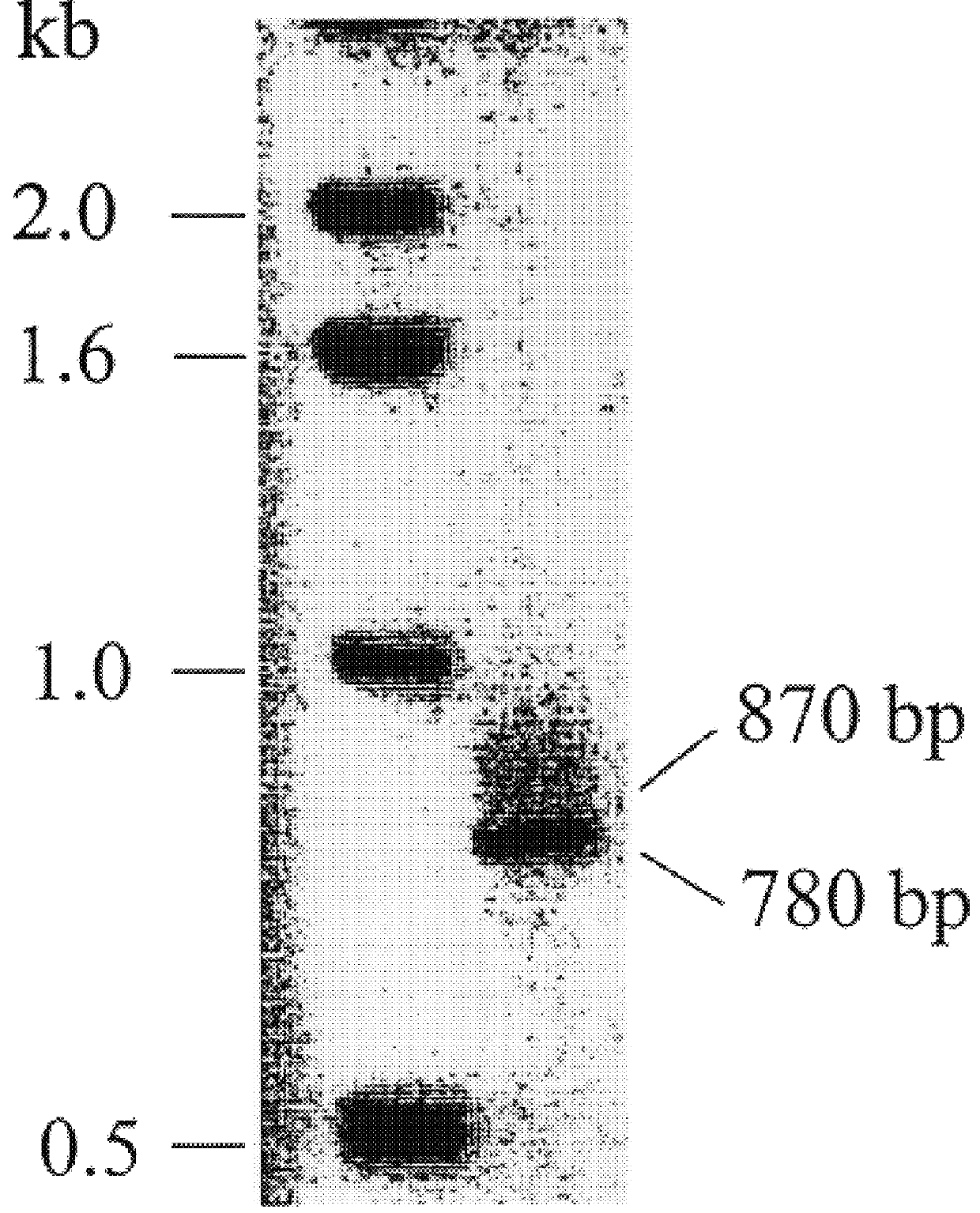
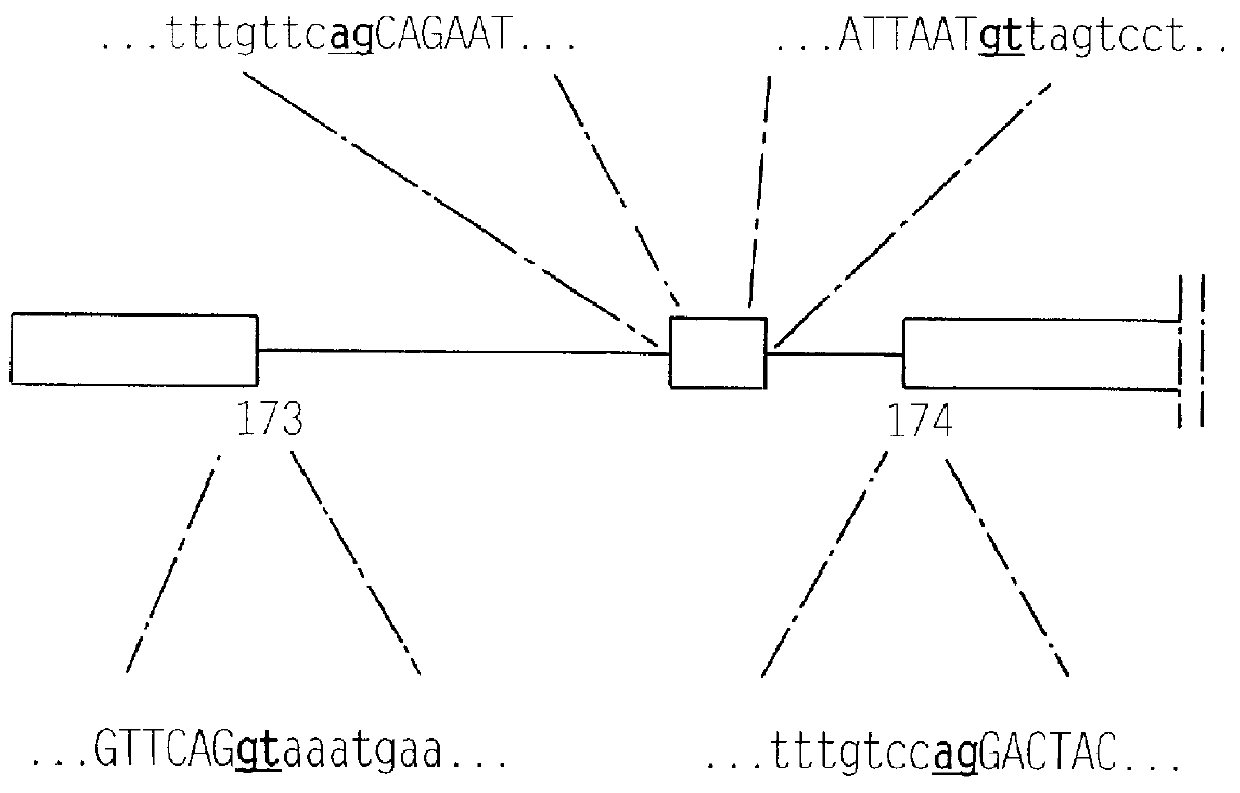
cDNA and genomic DNA cloning of MMP8alt
Cell culture
Two monocytic cell lines U937 and THP-1 cells were maintained in RPMI-1640 medium with 10% fetal bovine serum (Life Technologies, Inc.). Human chondrocytes were prepared from cartilage taken from patients undergoing joint replacement as described, Aydelotte M. B. and Kuettner K. E., (1988) Connect. Tissue Res. 18:205-222, and cultured in Dulbecco's modified medium with 10% fetal bovine serum.
cDNA and genomic DNA cloning
Total RNA isolation from THP-1 cells, U937 cells and human chondrocytes was accomplished using TriZol reagent (Life Technologies, Inc.). The RNA was reverse transcribed using the 1st Strand cDNA Synthesis Kit (Life Technologies, Inc.) and oligo-dT primer. PCR amplification of MMP-8 cDNA fragment corresponding to nucleotides 68 to 850 of the published sequence (GenBank Accession No. J05556) was performed with Pfu polymerase (Stratagene) for 30 cycles (95.degree. C., 1 min; 55.degree. C., 2 min; 72.degree. C., 3 min) us...
example 2
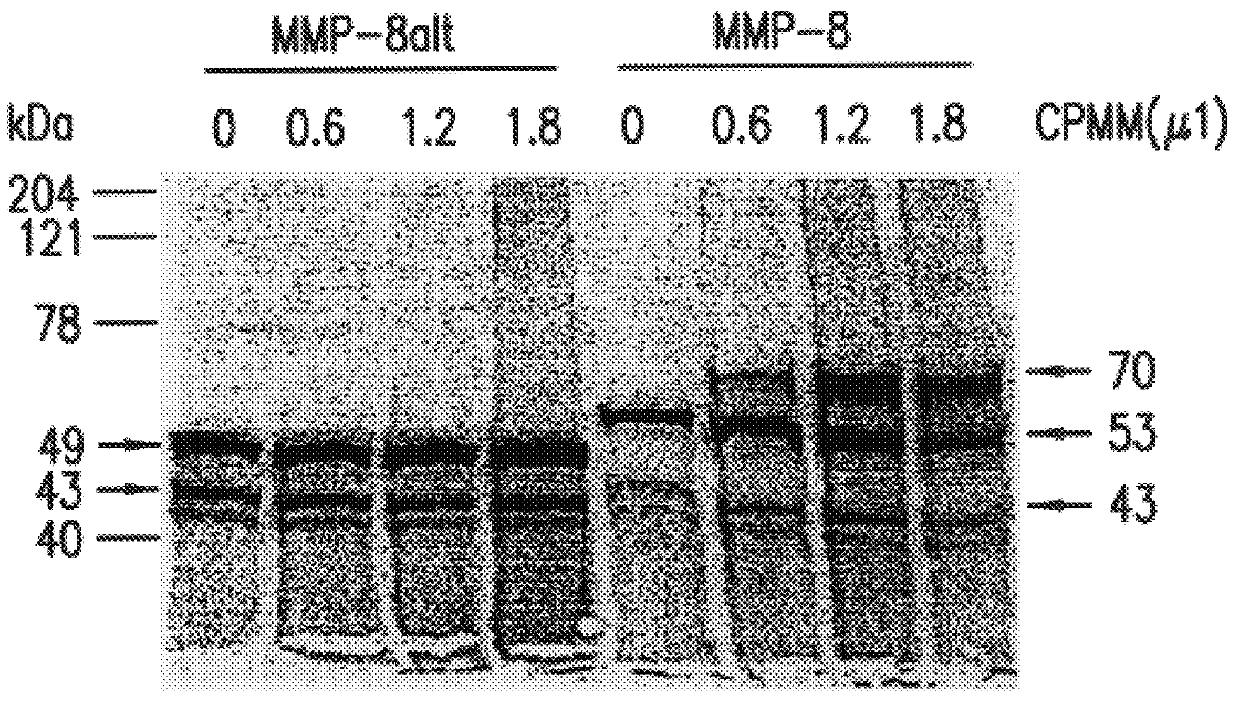
Assay for In vitro translation and Autoactivation of MMP-8 and MMP-8alt
In vitro translation of MMP-8alt cDNA is subcloned into pCDNA3+ vector is accomplished using TNT T7 Coupled Reticulocyte Lysate System (Promega) with .sup.35 S-methionine (Amersham). In certain cases, canine pancreatic microsomal membranes (Promega) are also included in the in vitro translation reaction to detect co-translational processing and glycosylation. Samples of in vitro translated proteins 50 ml) are diluted to 1 ml with 50 mM Tris-HCl, pH 7.5, 0.2 mM NaCl, 10 mM CaCl.sub.2, and 50 mM ZnCl.sub.2, and concentrated with a Centricon 10 (Amicon) to a final volume of 50 ml. Samples are then diluted 10 fold with the same buffer containing 0.05% Brij-35 and are activated by treatment with 2 mM p-aminophenylmercuric acetate for 90 min at 37.degree. C. In vito translated, .sup.35 S-labeled proteins are subjected to SDS-PAGE and autoradiography. The resultant sample sizes correspond to active MMP-8.
example 3
Assay for identifying MMP-8alt Substrates and Antagonists
An expression cloning procedure is used to identify potential physiological substrates of MMP-8alt. Wen, L.-P. et al., (1997) J. Biol. Chem. 272, 26056-26061; Kothakota, S. et al., (1997) Science 278, 294-298. A chondrocyte cDNA library is prepared in the expression vector pBK-CMV (Statagene). This library is subdivided into small pools in which each pool represents 100 cDNA clones. These pools of cDNA are in vitro transcribed and translated in the presence of .sup.35 S-methionine (Amersham) using the TNT T3 Coupled Reticulocyte Lysate System (Promega). Each translated pool is separated into two parts; one portion is incubated with MMP-8alt, and the other portion is incubated with heat inactivated MMP-8alt. The reaction products are resolved by SDS-PAGE and visualized by autoradiography. Potential substrates are identified by comparing the pattern of .sup.35 S-labeled proteins in the samples treated with active and inactive MM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com