Soluble polypeptide fractions of the LAG-3 protein, production method, therapeutic composition, anti-idiotype antibodies
a technology of soluble polypeptide fractions and lag-3 proteins, which is applied in the field of soluble forms derived from the lag3 membrane protein, can solve the problems of not enabling the detection of significant decreases in viral titres
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
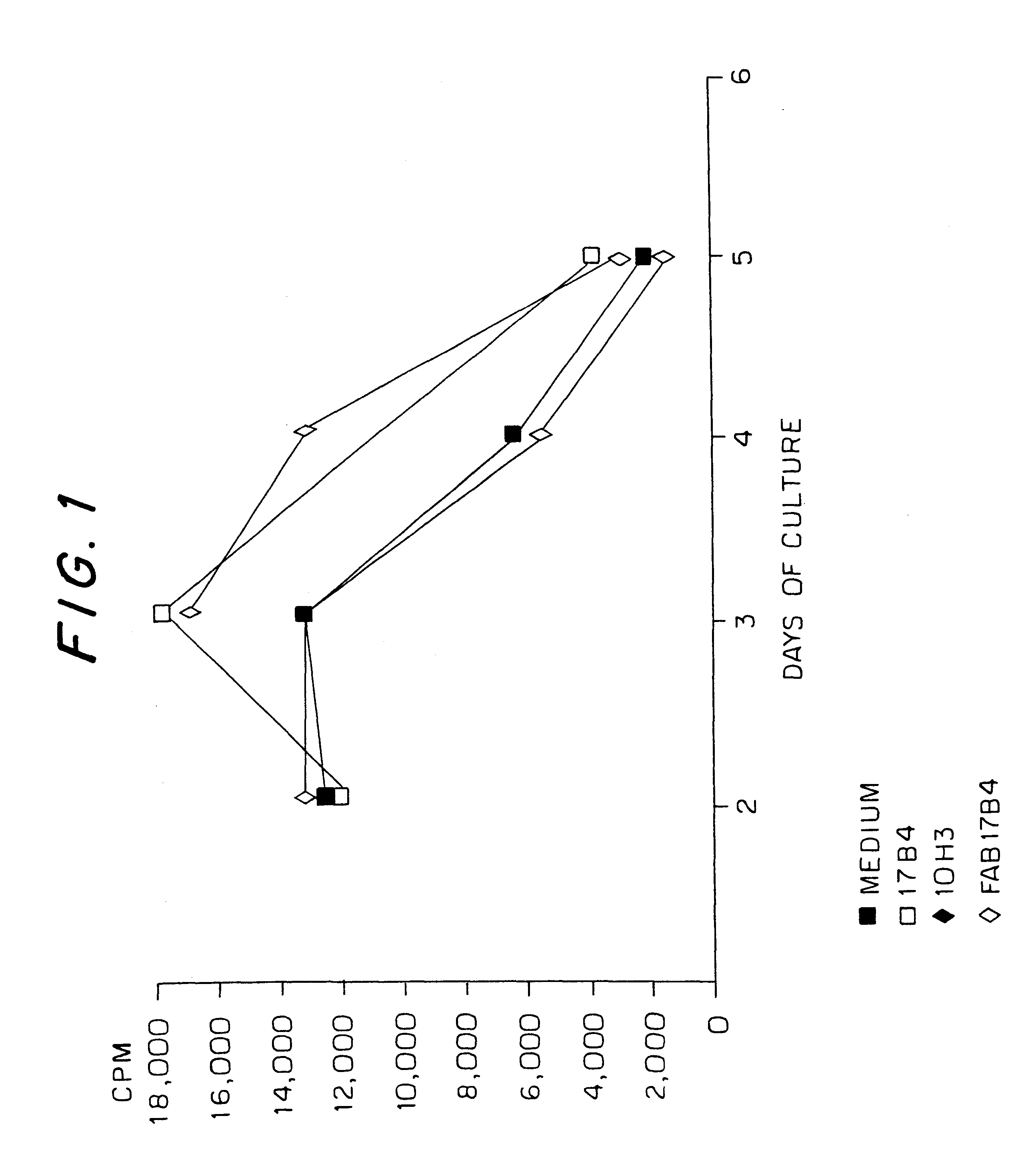
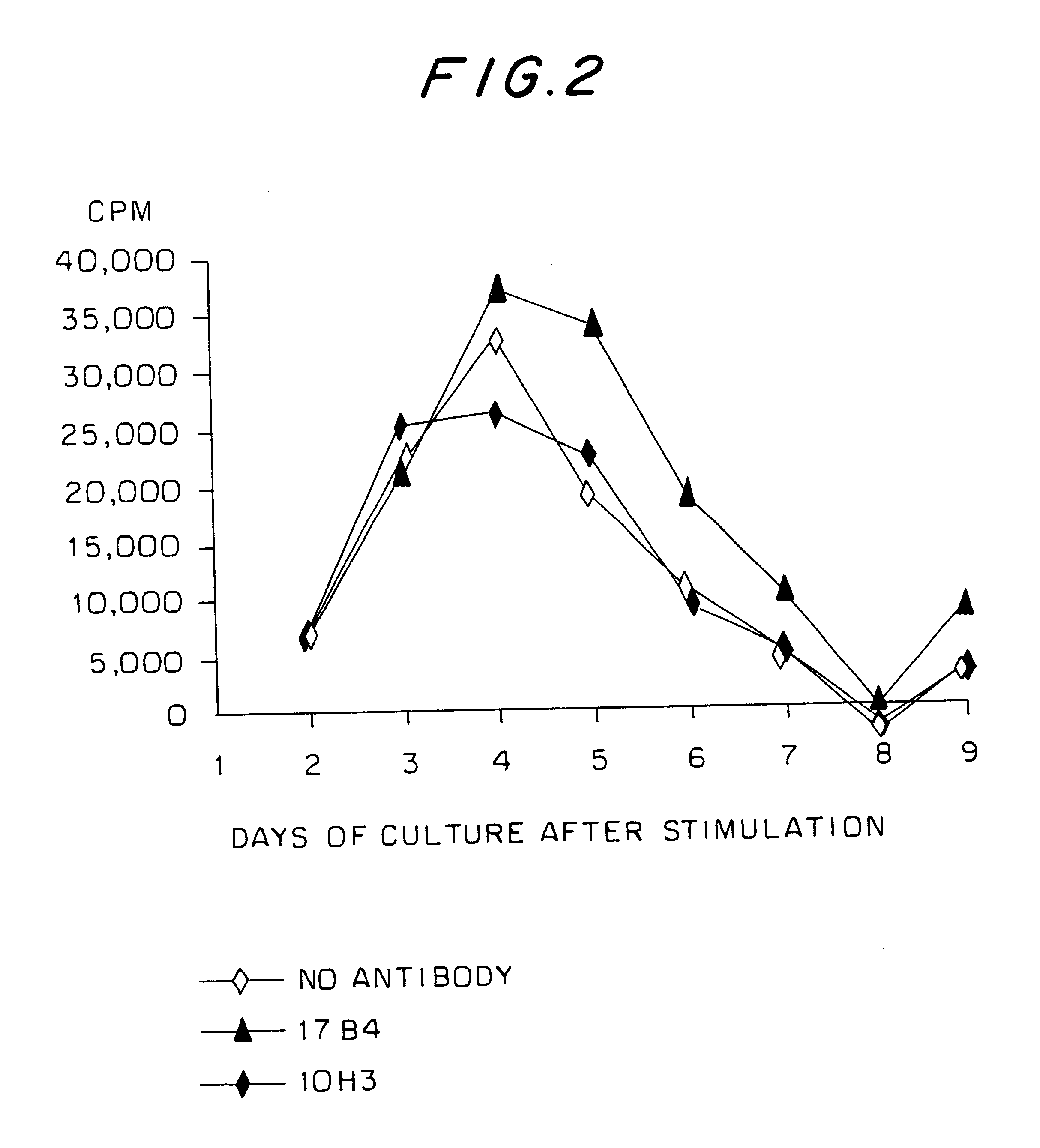
Proliferation of active T lymphocyte lines in the presence of anti-LAG-3 monoclonal antibodies
The anti-LAG-3 monoclonal antibodies used were 17B4, described in BAIXERAS et al. (2) and deposited at the CNCM under No. I-1240 on Jul. 10, 1992, and 11E3, described in HUARD et al. (8).
These antibodies belong to the isotype IgG1. These antibodies were tested for their biological effects on activated T lymphocytes, stimulated by specific antigenic peptides or processed antigens presented by MHC Class II molecules expressed by autologous antigen presenting cells, expressing LAG-3.
An anti-CD48 monoclonal antibody designated 10 H3 was used as irrelevant IgG1 antibody (negative control).
The saturating concentrations of anti-LAG-3 and anti-CD48 antibodies were determined by immunofluorescence on PHA (phytohaemagglutinin)-blasts and cell lines transformed by Epstein-Barr virus (EBV). In the proliferation tests, the monoclonal antibodies were added in the proportion of 5 times the saturating conc...
example 2
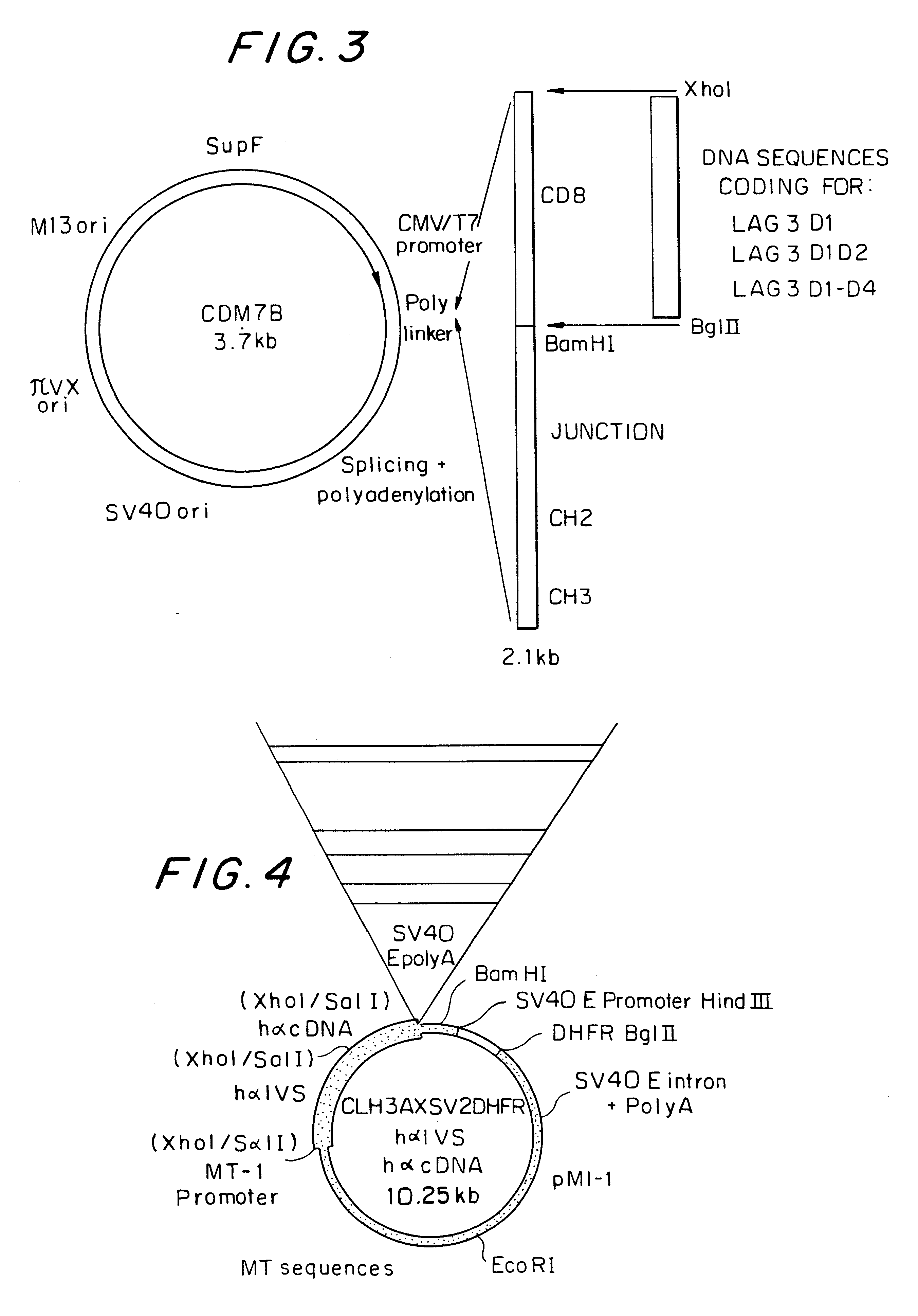
Transient expression of LAG-3 fusion proteins
Soluble proteins derived from LAG-3 were obtained by a recombinant DNA technique using suitable vectors comprising DNA coding for LAG-3 and DNA coding for an immunoglobulin fragment. The transient expression system consisted of transfected Cos cells. This system makes it possible to produce several mg of recombinant fusion proteins. Recombinant DNA techniques were carried out as described by MANIATIS et al. (22). The modifications were made as recommended by the manufacturer.
Construction of LAG-3 D1-D4 Ig and LAG-3 D1D2 Ig
Fragments coding for the D1D2 or D1-D4 regions were amplified (30 cycles) from a fragment of cDNA (FDC sequence) encompassing LAG-3 cDNA (TRIEBEL et al. (1)), using Taq polymerase free from 5'-endonuclease activity and relatively resistant to an exposure to very high temperature; the amplification was followed by a denaturation at 98.degree. C. (with a Perkin Elmer Cetus "DNA thermal cycle"). Specific primers were used a...
example 3
Production of soluble subfragments of LAG-3
In order to produce large amounts of recombinant proteins, a stable expression system consisting of transfected mammalian cells was developed. The host cells are anchorage-dependent hamster ovary (CHO) cells isolated from CHO cells deficient in dihydrofolate reductase (DHFR) and consequently necessitating glycine, a purine and thymidine for their growth. The pivotal role of DHFR in the synthesis of nucleic acid precursors, combined with the sensitivity of DHFR-deficient cells with respect to tetrahydrofolate analogues such as methotrexate (MTX), has two major advantages. Transfection of these cells with expression vectors containing the DHFR gene permits the secretion of recombinant DHFR-resistant clones, and the culturing of these cells on selective media containing increasing amounts of MTX results in amplification of the DHFR gene and the DNA associated therewith.
Construction of LAG-3 D1, LAG-3 D1D2, LAG-3 D1-D4
Fragments of DNA coding fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com