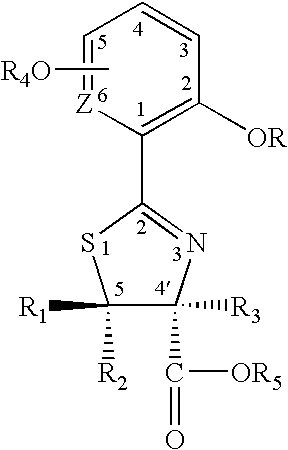
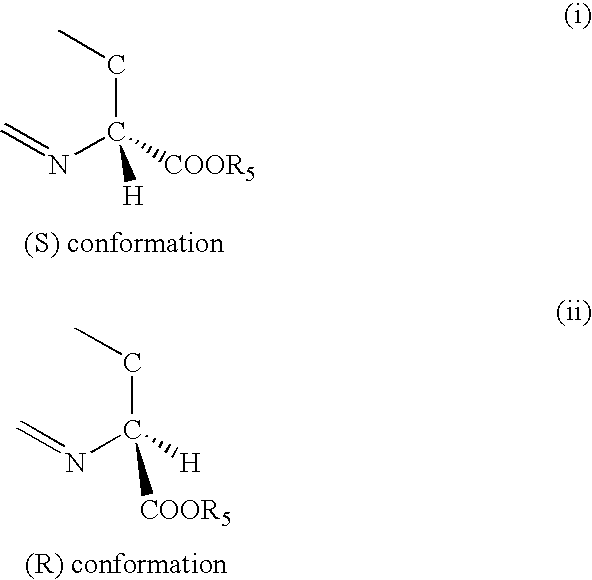
Thiazoline acid derivatives
a technology of thiazoline acid and derivatives, applied in the field of thiazoline acids, can solve the problems of inability to cyclic siderophores, low efficiency and marginal oral activity, and inability to tolerate cyclic siderophores,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
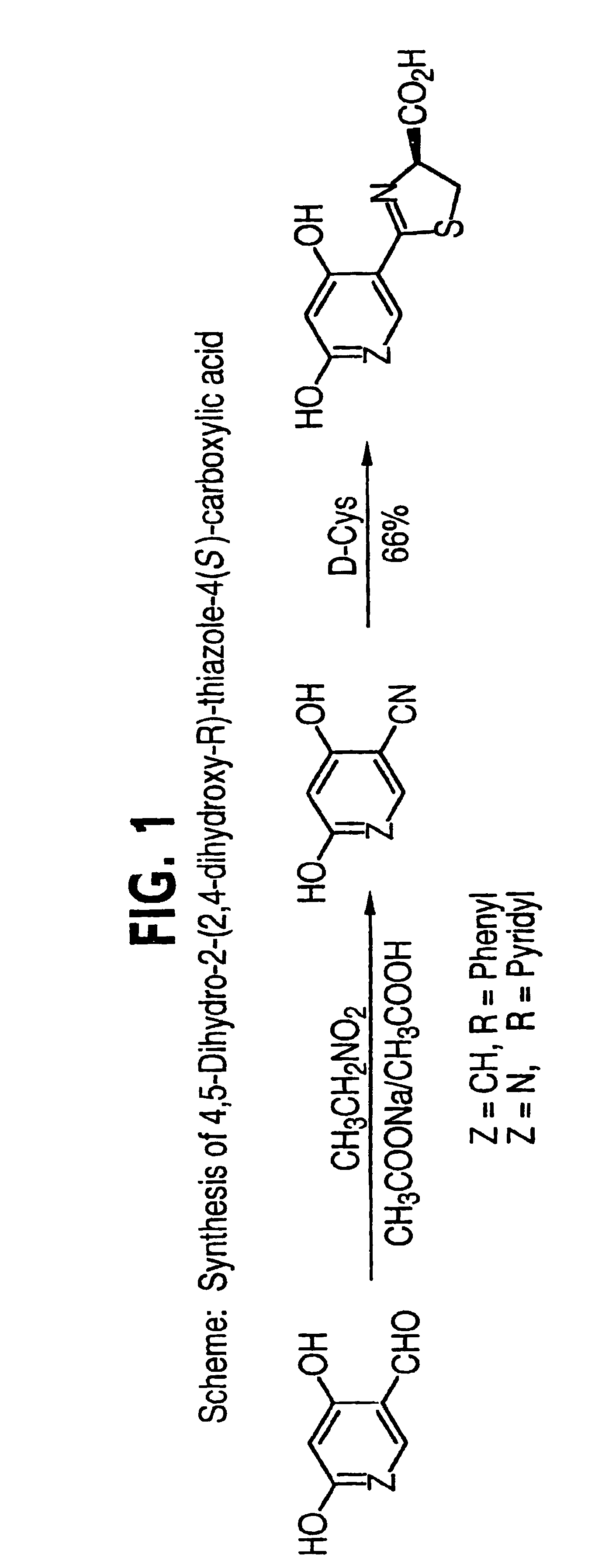
[0054]2,4-Dihydroxybenzonitrile was prepared according to the method of Marcus in Ber. disch. chem. Ges. 1981, 24, 3651, as follows:
[0055]A mixture of 2,4-dihydroxybenzaldehyde (5.0 g, 36.2 mmol), sodium acetate (5.94 g, 72.4 mmol), nitroethane (5.44 g, 72.4 mmol) and glacial acetic acid (10 ml) was refluxed for 6 hours. After cooling, the mixture was poured onto ice (100 g) and extracted with ethyl acetate (4×50 ml). The combined organic layers were washed with saturated NaHCO3 until the pH of the aqueous layer remained at 8, dried (Na2SO4) and the solvent removed in vacuo. Flash chromatography (SiO2cyclohexane:ethyl acetate=1:1) afforded 2,4-dihydroxybenzonitrile (2.87 g, 59%) as a pale yellow solid. 1H NMR (300 MHz, DMSO-d6) δ 6.33 (d, 1H, J=8.6 Hz), 6.43 (s, 1H), 7.37 (d, 1H, J=8.6 Hz), 10.35 (s, 1H), 10.78 (s, 1H), IR (KBc) 2200 cm−1.
example 2
[0056]4,5-Dihydro-2-(2,4-dihydroxyphenyl)-thiazole-4-(S)-carboxylic acid was prepared as follows:
[0057]D-cysteine hydrochloride monohydrate (6.8 g, 38.7 mmol) was added to a solution of 2,4-dihydroxybenzonitrile (3.5 g, 25.9 mmol) prepared in Example 1, in a mixture of degassed methanol (105 ml) and 0,2 To phosphate buffer, pH 5.95 (70 ml). NaHCO3 (3.25 g, 38.7 mmol) was carefully added and the mixture was slimed at 70° C. under Ar for 54 hours. Volatile components were removed under reduced pressure and the solution was acidified with 1 N NCL to pH 2. The resulting brown precipitate was vacuum filtered and the solid was washed with water (40 ml) and ethanol (20 ml). The crude product was dissolved in saturated NaHCO3 (700 ml) and the aqueous solution washed with ethyl acetate (2×200 ml). The aqueous layer was filtered through a fine frit and acidified with 1 N HCI to pH 2. The precipitated product was vacuum filtered. The aqueous layer was extracted with ethyl acetate (4×400 ml), t...
example 3
In Rats
[0060]Initial besting of 1 was performed in the non-iron-overloaded, bile duct-cannulated rat [J. Med. Chem., Vol. 34, Bergeron et al, “Synthesis and Biological Evaluation of Hydroxamate-Based Iron Chelators,” pages 3182-3187 (1991)]. The drug was prepared as a solution in 40% Cremophor-H2O and administered at a dose of 150 μmol / kg p.o. The rats were fasted for 24 hours before dosing. The efficiency of iron excretion induced by 1 was 2.4±0.92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com