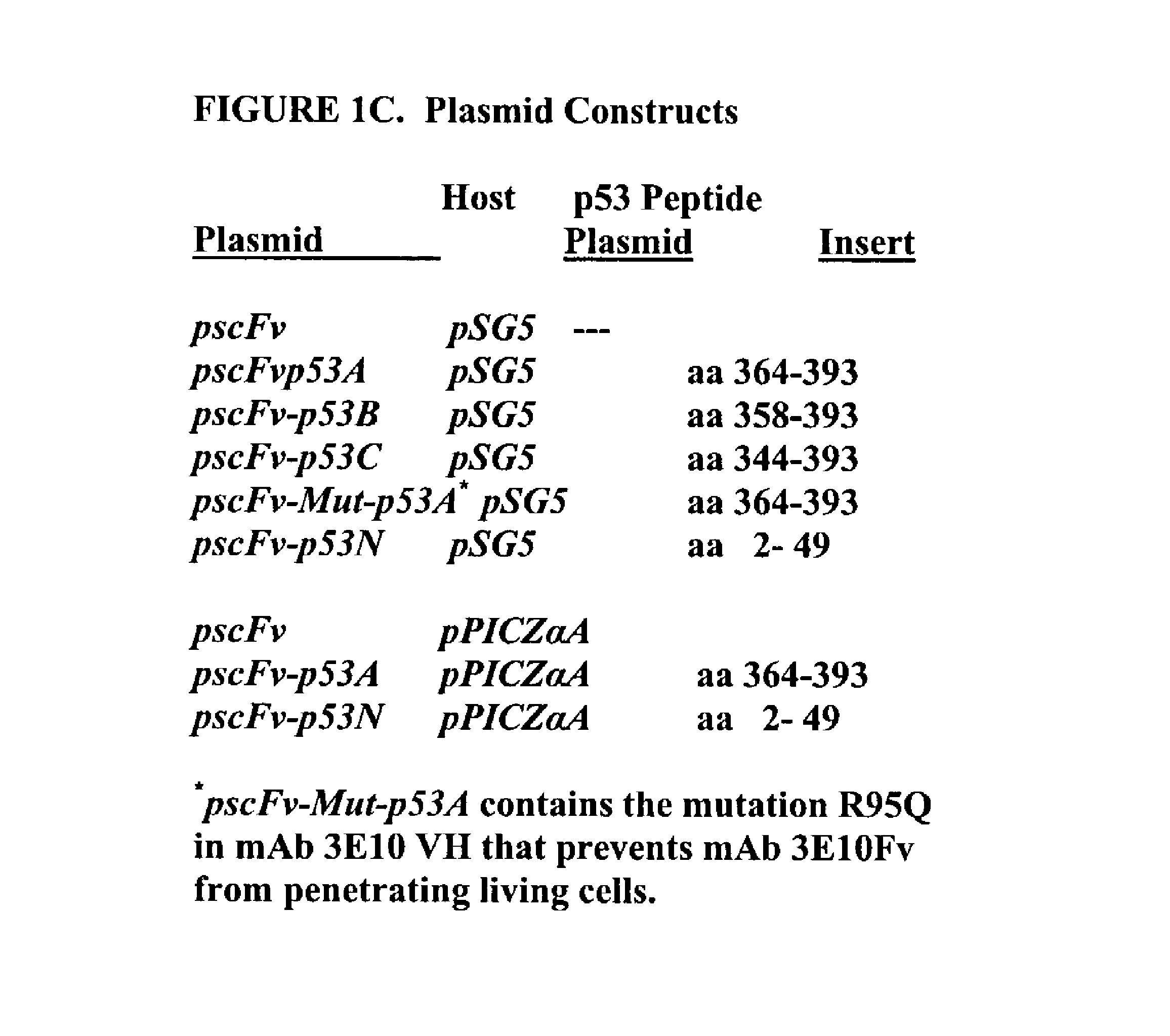
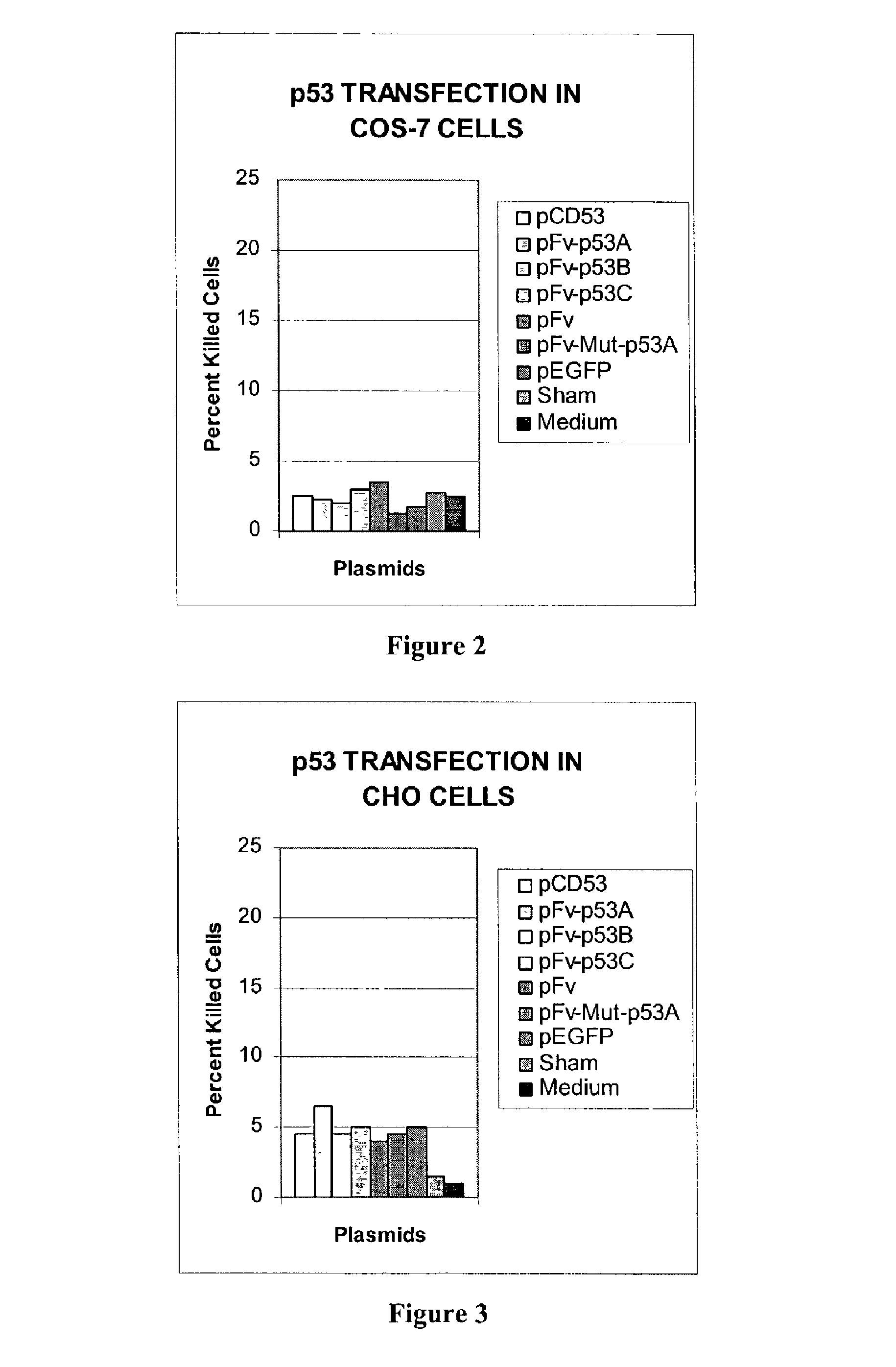
Delivery system using MAB 3E10 and mutants and/or functional fragments thereof
a delivery system and mab 3e10 technology, applied in the direction of peptide/protein ingredients, immunoglobulins, peptides, etc., can solve problems such as foreign proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Monoclonal Antibodies
[0065]mAbs 3E10, 5C6, and 4H2 are IgG2a anti-dsDNA antibodies which were produced from spleen cells of MRL-mpj / lpr mice by fusion with cells from the FOX-NY cell line as previously described (see Weisbart, et al., in J. Immunol. 144:2653 (1990)). mAb PP102 (Chemicon International, Temecula, Calif.), a murine IgG2a antibody that does not bind DNA, was used as a non-anti-DNA antibody isotype-matched control.
example 2
Monoclonal anti-DNA Antibody Binding in Vitro to Tissues of Human Organs
[0066]The monoclonal antibodies were purified from ascites by affinity chromatography using protein A-Sepharose and tested for binding kidney. mAb 3E10 was also tested for the ability to bind tissues from 19 other human organs, including blood vessels, nerve trunks, liver, connective tissues, lung, pancreas, gut, cardiac muscle, striated muscle, spleen, ovary, testis, thyroid, skin, eye, adrenal, brain, pituitary, and bone. Binding of monoclonal antibodies was detected with peroxidase conjugated affinity purified rabbit antibodies specific for mouse IgG Fc as previously described (see Taylor and Lote in Immunomicroscopy: A diagnostic tool for the surgical pathologist. Saunders, W. B., Philadelphia (1994)).
mAb 3E10 Binds Human Renal Tubular Cells in Vitro
[0067]Three anti-dsDNA antibodies, i.e., mAbs 3E10, 5C6, and 4H2, were studied in vitro for binding tissue from fixed normal human kidney. All of the antibodies ...
example 3
Monoclonal anti-DNA Antibody Binding in Vivo to Tissues of Normal BALB / c Mice
[0071]Normal BALB / c mice were primed with pristane and injected intraperitoneally with 2×107 hybridoma cells. After two weeks, the animals developed ascites containing antibodies with anti-dsDNA reactivity. Heart, liver, and kidney tissues were obtained and preserved in liquid nitrogen for studies of tissue histology. Binding of the anti-DNA antibodies to tissues was detected with peroxidase-conjugated affinity-purified rabbit antibodies specific for mouse IgG Fe as previously described (see Taylor and Lote supra).
mAb 3E10 Binds Murine Renal Tubular Cells in Vivo
[0072]Thus, to determine if mAb 3E10 was reactive with mouse renal tubular cells in vivo, kidneys were examined from normal BALB / c mice two weeks after intraperitoneal injection with 3E10 cells to establish ascites containing high concentrations of mAb 3E10. mAb 3E10 did not localize in the membrane, cytoplasm, or nuclei of liver, cardiac muscle, or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com