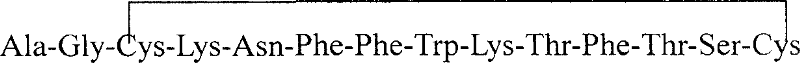Somatostation a aqua prepn and its prepn process and application
A technology of somatostatin and aqueous solution, which is applied in blood diseases, pharmaceutical formulations, aerosol delivery, etc., and can solve problems such as limitations, single dosage forms, and increased drug pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. Preparation of the pharmaceutical preparation according to claim 7
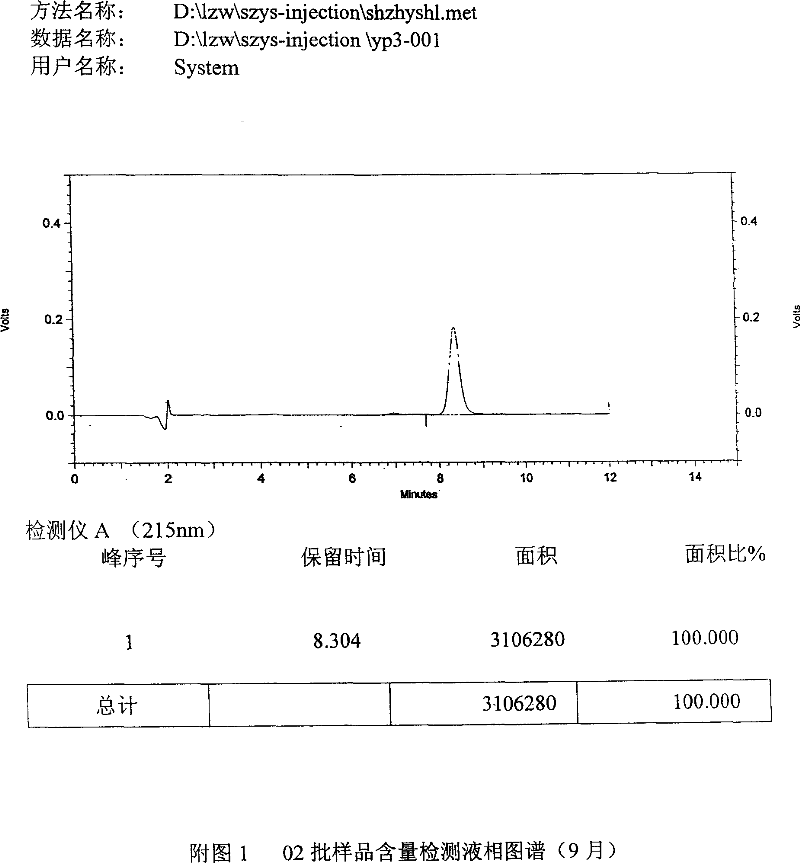
[0055] Precisely weighed sodium chloride 7.0g, disodium hydrogen phosphate (containing 12 crystal water) 4.37g, sodium dihydrogen phosphate (containing 2 crystal water) 1.22g, disodium edetate 1.28g, somatostatin 3.0g , Dissolve in water for injection, and finally dissolve to 1000ml, then use 2ml vials to dispense into 1000 pieces. The final specification is 3mg / bottle. According to this prescription, a total of 3 batches of samples were produced, numbered 01, 02, and 03 respectively. The operating procedure is carried out in accordance with the description of the manual.
Embodiment 2
[0056] Example 2. Preparation of the pharmaceutical preparation according to claim 10
[0057] Precisely weighed sodium chloride 7.0g, disodium hydrogen phosphate (containing 12 crystal water) 4.37g, sodium dihydrogen phosphate (containing 2 crystal water) 1.22g, disodium edetate 1.28g, somatostatin 3.0g , Hydroxypropyl β-cyclodextrin 20g, dissolved in water for injection, and finally dissolved to 1000ml, divided into 1000 with 2ml vials. The final specification is 3mg / bottle. A total of 1 batch of samples were produced according to the prescription, number 04. The operating procedure is carried out in accordance with the description of the manual.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com