Targeted small interference RNA formulation for treating viral Hepatitis B and its preparation
A hepatitis B, small interference technology, applied in antiviral agents, gene therapy, pharmaceutical formulations, etc., can solve problems such as low cationic load, achieve good protection, increase drug concentration, and inhibit virus replication and infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of linear nucleic acid-binding polypeptides.
[0041] Synthesize single-stranded DNA sequences with overlapping ends of the following adjacent fragments,
[0042] 5'aagaaggccttattggcgttcgccttcgcc'3; 5'caccacctcgcgctcctcgcgctcctcgcgcaccacctcgc'3; 5'gctcgcgctccaccacctggcgctcgccctt'3 were dissolved in appropriate amount of water, ligated into double-stranded DNA molecules under the action of T4 DNA ligase, and cloned into the yeast expression vector pICZα, verified by enzyme digestion and sequenced After verification, the following procedure was used to introduce the expression vector into Pichia pastoris cells.
[0043] First, the DNA of the yeast expression vector pICZα was cut with Sac1 for more than 1 hour to linearize it. Make up water to 300ul, add 300ul phenol: chloroform: isoamyl alcohol (25:24:1) and mix by inversion. Centrifuge at 12000 rpm for 5 minutes. Take the supernatant, add 750ml ethanol, 30ul NaAc, -20°C, 30 minutes. Centrifuge,...
Embodiment 2
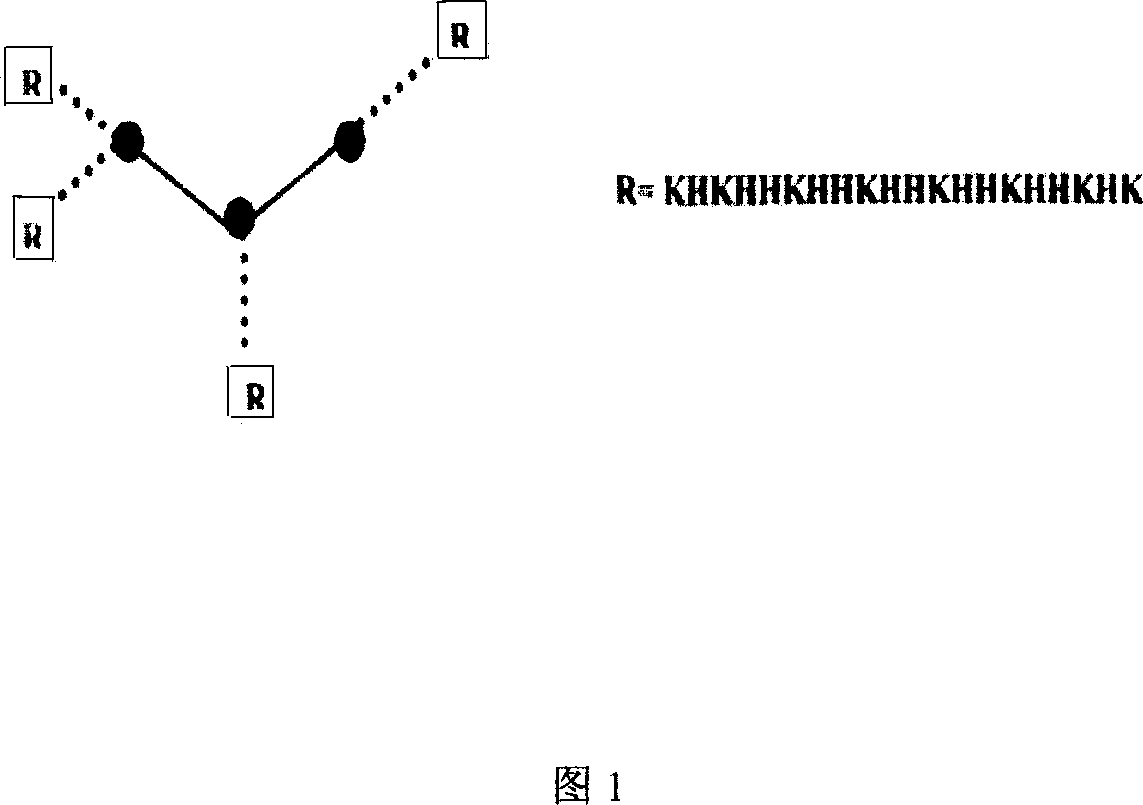
[0057] Example 2: Preparation of branched nucleic acid-binding polypeptides.
[0058] Branched peptide synthesis can be carried out on the ABI431A solid-phase automatic peptide synthesizer or manually synthesized. The sequence structure is shown in Figure 1. Adopt standard Fmoc method, select 0.125mmol Fmoc-MAP-Branch-PSC resin, make peptide chain extend from C terminal to N terminal one by one according to sequence, the dosage of each amino acid is 0.5mmol, amino acid: resin=4: 1, the first Amino acid was connected to the resin with DMAP, the activation of amino acid was coupled with HOBt and DCC, and the Fmoc protecting group was removed with 20% piperidine solution. After the polypeptide is synthesized, according to the steps recommended by PE company, add the compound after ice bath to the 10ml cutting solution under the condition of ice bath, use cutting solution B, its components are 0.75mg of crystalline phenol, 0.25ml of EDT, 0.5ml of sulfide anisole. Ionized water 0....
Embodiment 3
[0059] Example 3: Preparation of lactosylated or galactosylated nucleic acid-binding polypeptides.
[0060] Take 200 mg of the polypeptide prepared above, 800 mg of α-lactose, and 500 mg of sodium cyanoborate and dissolve them in 200 ml of water. After reacting at 37°C for 24 hours, adjust the reaction solution to pH 8.5 with 5mol / L KOH solution, and continue to react at 37°C for 6 hours. . The reaction solution was dialyzed with water at room temperature to terminate the reaction to remove unreacted lactose, and the sulfate-allthone method was used for qualitative detection until the lactose content in the dialysate was negative, and the product was vacuum-dried for later use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com