Zinc oxide hollow microsphere preparation method
A hollow microsphere, zinc oxide technology, applied in zinc oxide/zinc hydroxide and other directions, can solve the problem of rare reports on the preparation and research of zinc oxide hollow microspheres, and achieve good catalytic performance and fluorescence emission performance, photocatalytic performance. The effect of increased activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] In a beaker, 10 grams of sodium-type strong-acid cation-exchange resins are soaked 2 times in 250 milliliters of weight percentages in 8% sulfuric acid solution, each 2 hours, completely replace other cations in the sodium-type strong-acid cation-exchange resins with hydrogen ions, and then use After fully washing with deionized water and drying, the hydrogen-form strong acid cation exchange resin can be obtained. Add 10 grams of hydrogen-type strong acid cation exchange resin to 250 ml of 0.5M zinc nitrate solution in a beaker, M is the molar concentration, and shake it on a shaker for 6 hours to make the zinc ion and hydrogen-type strong acid cation exchange resin The hydrogen ions are fully exchanged, and then filtered; the exchanged and filtered hydrogen-type strong acid cation exchange resin is put into an oven and dried at a temperature of 100°C for 2 hours to remove moisture; then roasted in a muffle furnace at 730°C for 4 hours, that is About 3.9 grams of zinc o...
Embodiment 2
[0013] In a beaker, 10 grams of sodium-type strong-acid cation-exchange resins are soaked 2 times in 250 milliliters of weight percentages in 8% hydrochloric acid solution, each 2 hours, completely replace other cations in the sodium-type strong-acid cation-exchange resins with hydrogen ions, and then use After fully washing with deionized water and drying, the hydrogen-form strong acid cation exchange resin can be obtained. Add 10 grams of hydrogen-type strong acid cation exchange resin to 250 milliliters, 0.4M zinc acetate solution in a beaker, and place it on a shaker for 6 hours, so that the zinc ions and the hydrogen ions on the hydrogen-type strong acid cation exchange resin are fully Exchange and then filter; put the exchanged and filtered hydrogen-type strong acid cation exchange resin into an oven and dry at 60°C for 10 hours to remove moisture; then roast in a muffle furnace at 900°C for 2 hours to obtain 3.8 grams Left and right zinc oxide hollow microspheres. Dete...
Embodiment 3
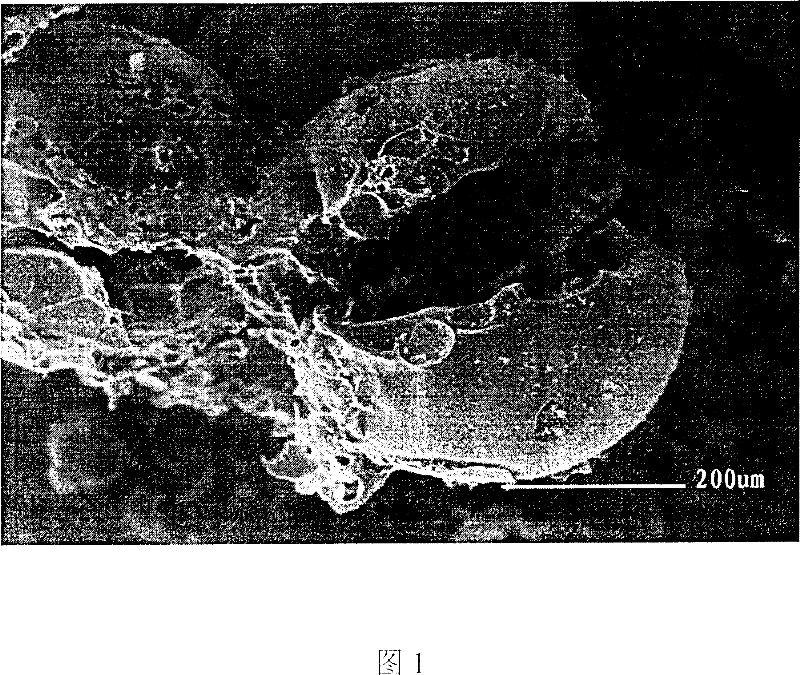
[0015] Add 10 grams of sodium-type strong acid cation exchange resin to 250 ml of 0.5M zinc sulfate solution in a beaker, and shake it on a shaker for 5 hours to fully exchange zinc ions with sodium ions on the sodium-type strong acid cation exchange resin , and then filtered; the exchanged and filtered sodium-type strong acid cation exchange resin was put into an oven and dried at a temperature of 90°C for 6 hours to remove moisture; then roasted in a muffle furnace at 600°C for 5 hours to obtain about 3.7 grams Zinc oxide hollow microspheres. Determination results: The prepared zinc oxide hollow microspheres were analyzed by Shimadzu-6000 X-ray diffractometer for the crystal form of zinc oxide, and the surface morphology of the microspheres was observed by the XL30 scanning electron microscope of Philips Company in the Netherlands. The results showed that the prepared zinc oxide microspheres The balls are hollow, with a diameter of about 300-500 μm, belonging to the hexagona...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com