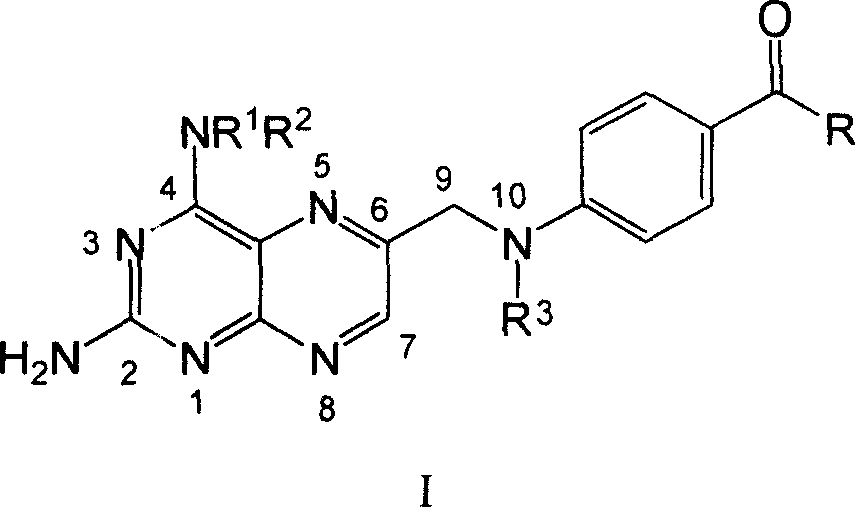
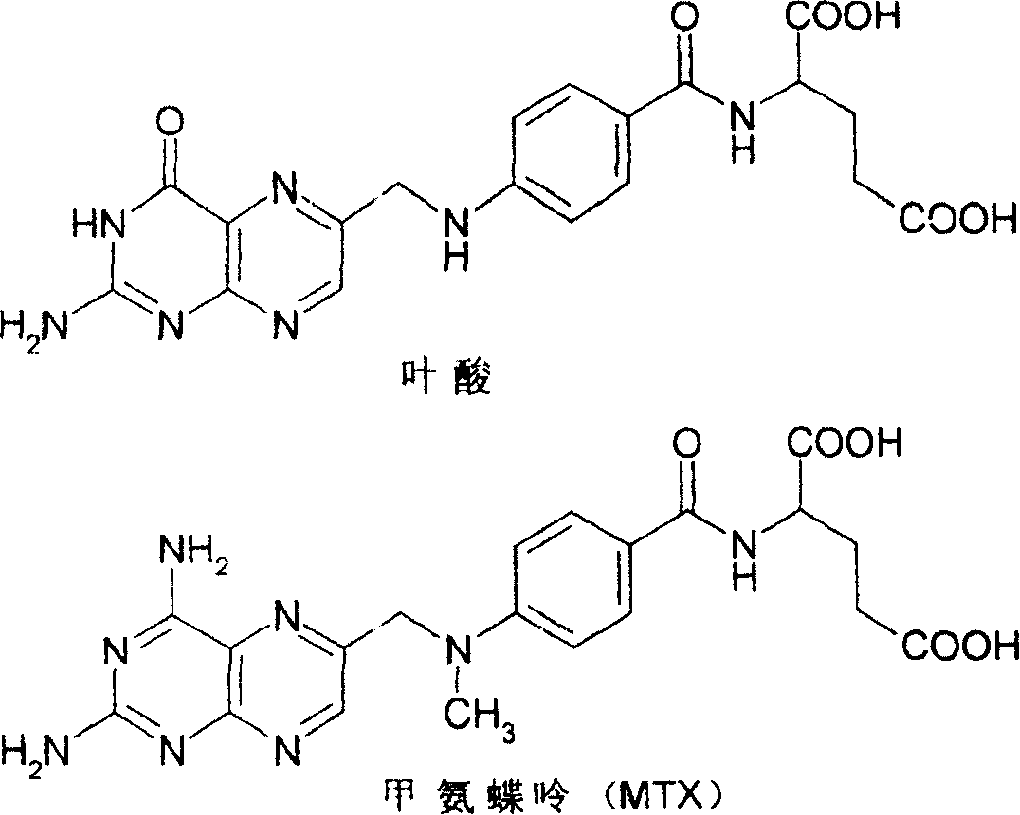
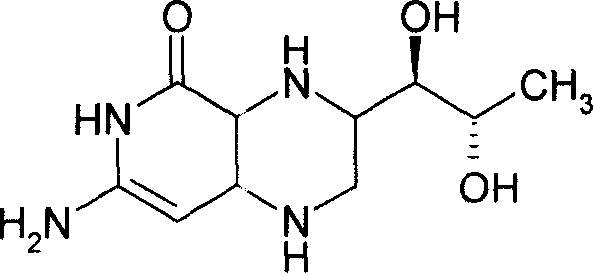
Methylamino-pterin derivative with inhibiting nitric oxide synthetase function
A technology of compounds and derivatives, applied in the field of new MTX derivatives, can solve the problems of high toxicity, narrow anti-cancer spectrum, and expanding anti-cancer spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0074] 1. Instruments and reagents:
[0075] The melting point of each compound in this experiment was determined by Mel-TEMP melting point apparatus, and the temperature was not corrected. MS was determined by HP1100LC / MSD mass spectrometer. Silica gel GF for thin layer chromatography (TLC) plates 254 (Qingdao Ocean Chemical Factory) and 0.8% CMC-Na distilled aqueous solution are stirred evenly and then paved, then activated at 100-110°C for 1h, placed in a desiccator for future use, and placed under an ultraviolet lamp (wavelength 254nm and 365nm) for color development; column chromatography adopts 100-200 or 200-300 mesh silica gel (produced by Qingdao Ocean Chemical Factory), and the column is packed by dry method. The H-NMR spectrum is measured with a Bruck AV-300 nuclear magnetic resonance instrument, without special instructions, the solvent used is D 6 -DMSO. Elemental analysis was determined with an Elementar Vario EL III instrument. Reagents are commercially ava...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com