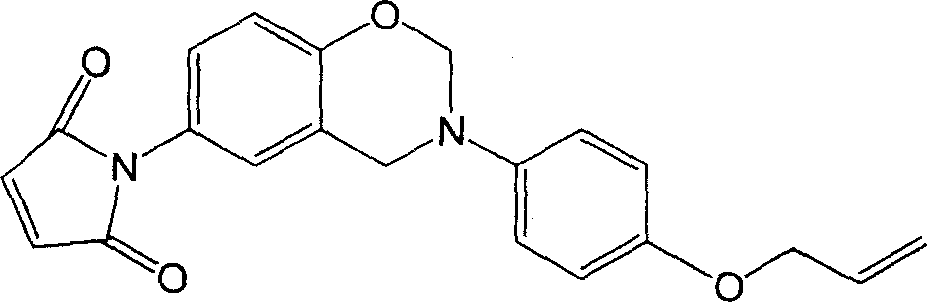
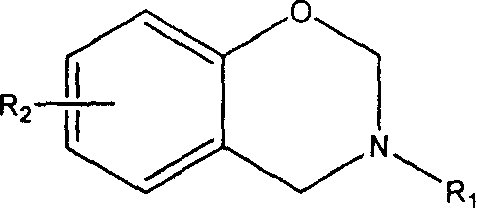
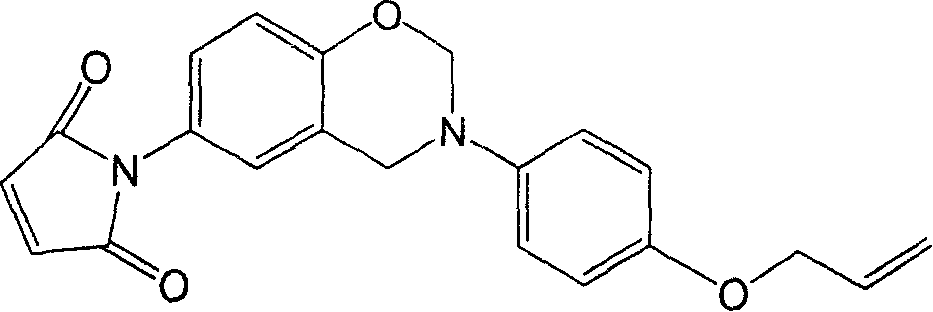
Benzoxazine containing maleimide and allyl ether and its preparing method
A technology of maleimide and benzoxazine, which is applied in the field of benzoxazine containing maleimide and allyl ether and its preparation, can solve the problem of unfavorable forming process and processing temperature window Insufficient width and other problems, to achieve the effects of low production cost, easy molding and processing, and improved room temperature fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 7.46g (0.05mol) 4-(allyloxy)aniline and 1.50g (0.05mol) paraformaldehyde into the reactor, stir and react at 100°C for 60min, then completely dissolve the reactant in chloroform, and use 1N NaOH solution was washed 5 times, then washed with deionized water to neutrality, filtered and dried to obtain the intermediate product; 7.57g (0.04mol) 4-hydroxyphenylmaleimide, 1.20g ( 0.04mol) of paraformaldehyde, heated up to 90°C and stirred for 2 hours. After the reaction was stopped, chloroform was added until the reactants were completely dissolved, and then 10% NaHCO 3 After the solution was washed 3 times, it was washed with deionized water until neutral, filtered and dried to obtain 10.37 g of the product with a yield of 57.2%.
[0027] The melting temperature of this product measured by DSC (differential scanning calorimetry, test condition heating rate 5°C / min, N2 atmosphere, 60ml / min) is 114°C; TGA (thermogravimetric analysis, test condition heating rate 5°C / min, ...
Embodiment 2
[0032] Add 5.97g (0.04mol) 4-(allyloxy)aniline and 1.80g (0.06mol) paraformaldehyde in the reactor, stir and react at 90°C for 55min, then completely dissolve the reactant in chloroform, and use 3N NaOH solution was washed 3 times, then washed with deionized water to neutrality, filtered and dried to obtain the intermediate product; 3.31g (0.033mol) 4-hydroxyphenylmaleimide, 1.05g ( 0.035mol) of paraformaldehyde, heated up to 120°C and stirred for 6 hours. After the reaction was stopped, chloroform was added until the reactants were completely dissolved, and then 5% NaHCO 3 After the solution was washed 4 times, it was washed with deionized water until neutral, filtered and dried to obtain 8.46 g of the product with a yield of 58.4%.
Embodiment 3
[0034] Add 14.92g (0.10mol) of 4-(allyloxy) aniline and 5.40g (0.18mol) of paraformaldehyde into the reactor, stir and react at 85°C for 35min, then completely dissolve the reactant in ethyl acetate , washed 4 times with 2N NaOH solution, then washed with deionized water until neutral, filtered and dried to obtain the intermediate product; in the intermediate product, add 17.03g (0.09mol) 4-hydroxyphenylmaleimide, 2.70 g (0.09mol) of paraformaldehyde, heated up to 110°C and stirred for 4 hours. After the reaction was stopped, ethyl acetate was added until the reactant was completely dissolved, and then 3% KHCO 3 After the solution was washed 5 times, it was washed with deionized water until neutral, filtered and dried to obtain 22.86 g of the product with a yield of 63.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com