Method for controlling biooxidation reactions
A biooxidation and biocatalyst technology, applied in fermentation and other fields, can solve the problems of high cost, increased risk of batch damage, cumbersome real-time control of nutrient feeding, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
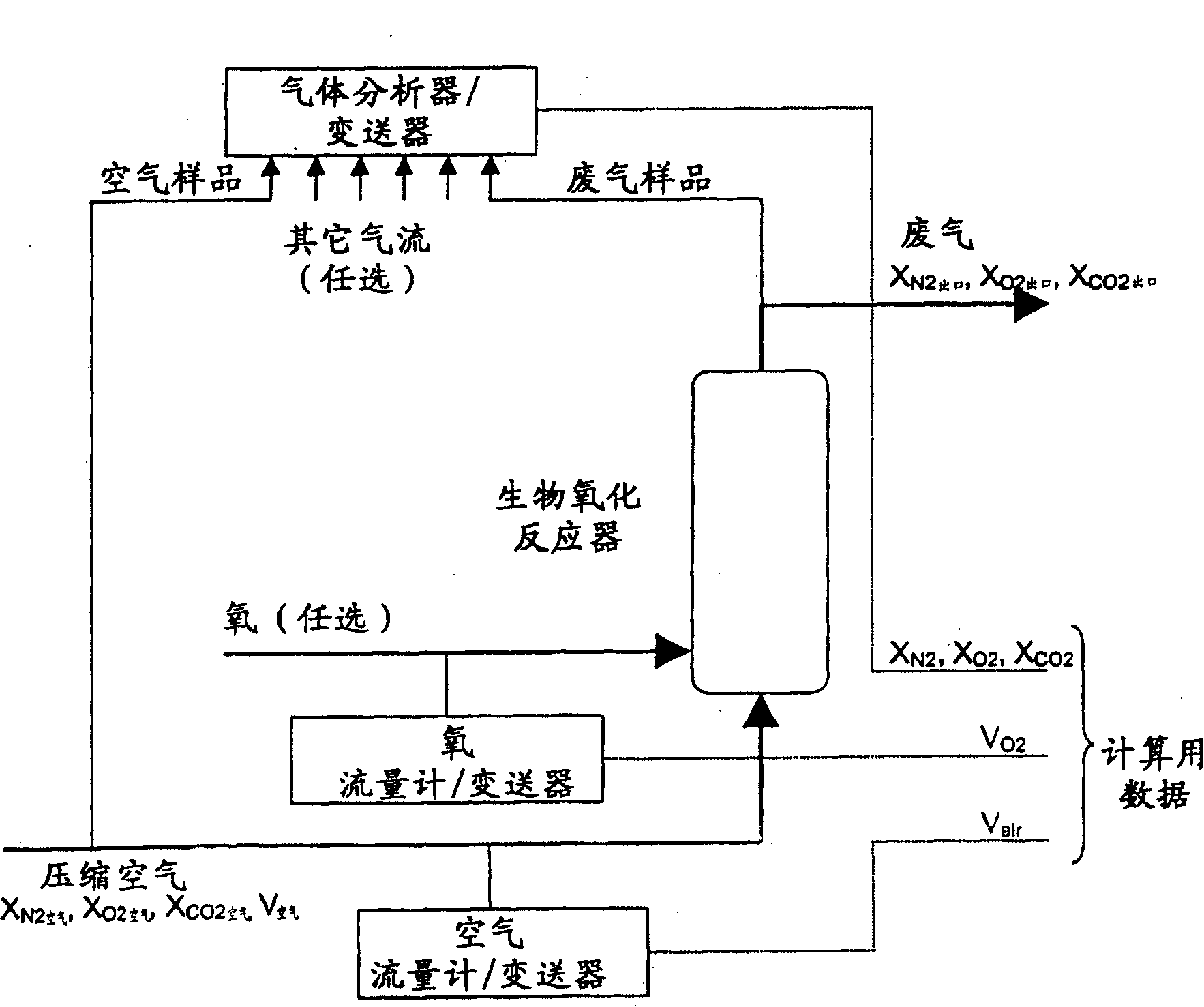
[0097] Composite gas balance calculations using optional oxygen supplementation
[0098] The purpose of this example is to provide the r CO2 ·V f and -r O2 ·V f way of expression. The mathematical formulas implemented in determining the rates of carbon dioxide evolution and oxygen uptake used in the methods of the present invention are currently derived from gas phase compositional balances. A simplified flow chart of the gas flow system of the biooxidation reactor used to determine the compositional gas balance calculation is attached figure 1 , where an oxygen-containing gas, usually air, is sparged into the reaction mixture. The supplemental supply of oxygen is optionally blended with air to enrich the oxygen content or sprayed directly into the biooxidation reactor. The constituent gas balance equations are correct and are also algebraically independent of the feed location where air or optional oxygen is provided. The combined gas is contacted with a partially oxy...
Embodiment 2
[0118] Composition Gas Balance Measuring Device
[0119] Gas flow measurements are performed using any number of gas flow devices that generate calibrated analog or digital signals representative of gas flow rates. These devices include thermal mass flow meters, orifice flow meters and coriolus effect meters. These instruments typically express gas flowerets in standard volumetric units at certain standard temperatures and pressures. These are the standard T° and P° used in the method of Example 1. Instrument sellers may also provide K values if the flowmeter is used with a gas other than the calibration gas.
[0120] Various instruments can be used for gas composition analysis in the method of Example 1. Where common air supplies can be fitted to multiple biooxidation reactors, the most useful is to sample the compressed air supply and biooxidation in repeated sequences to common instruments. A mass spectrometer is the preferred measuring device due to its ability to si...
Embodiment 3
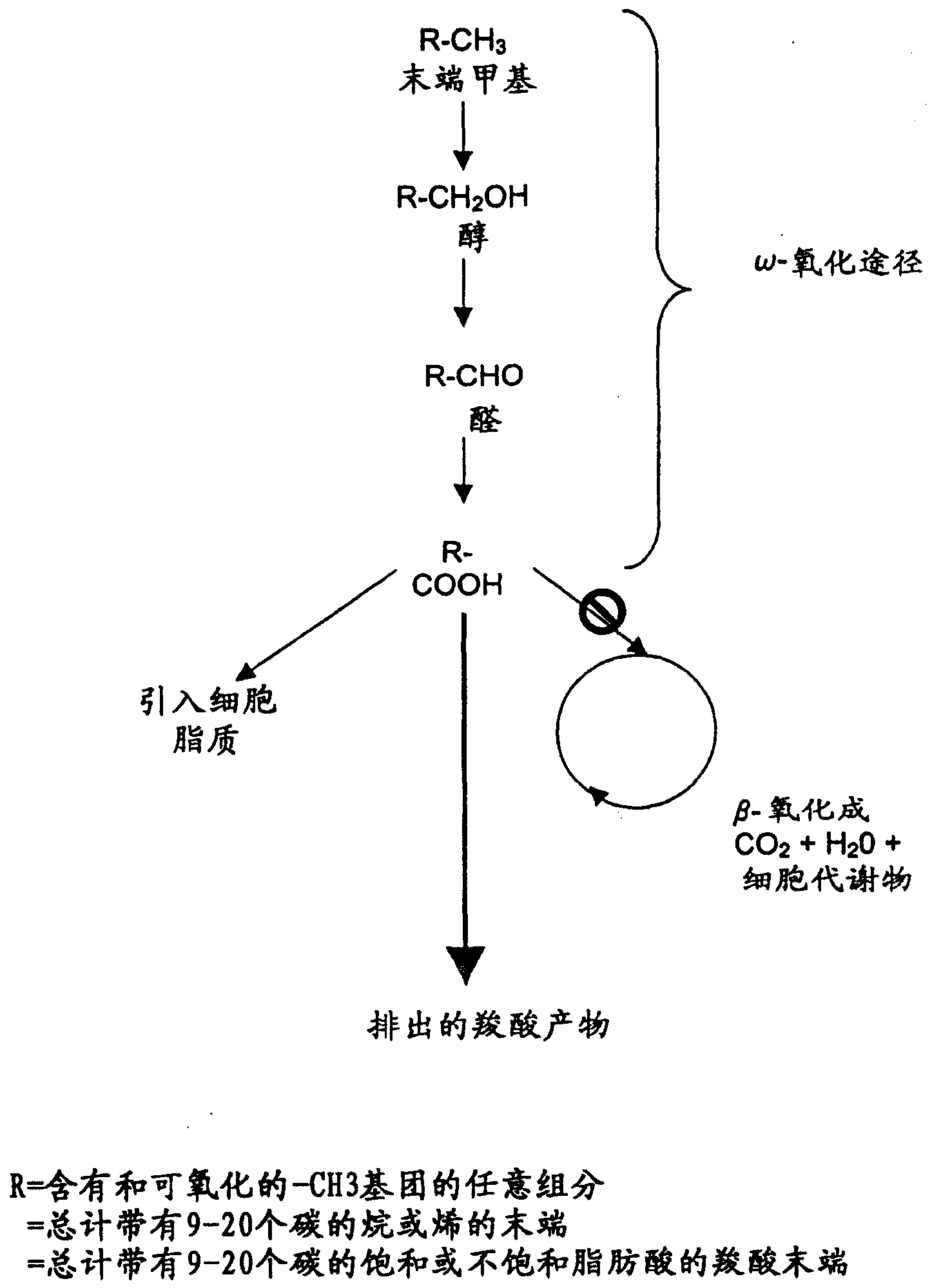
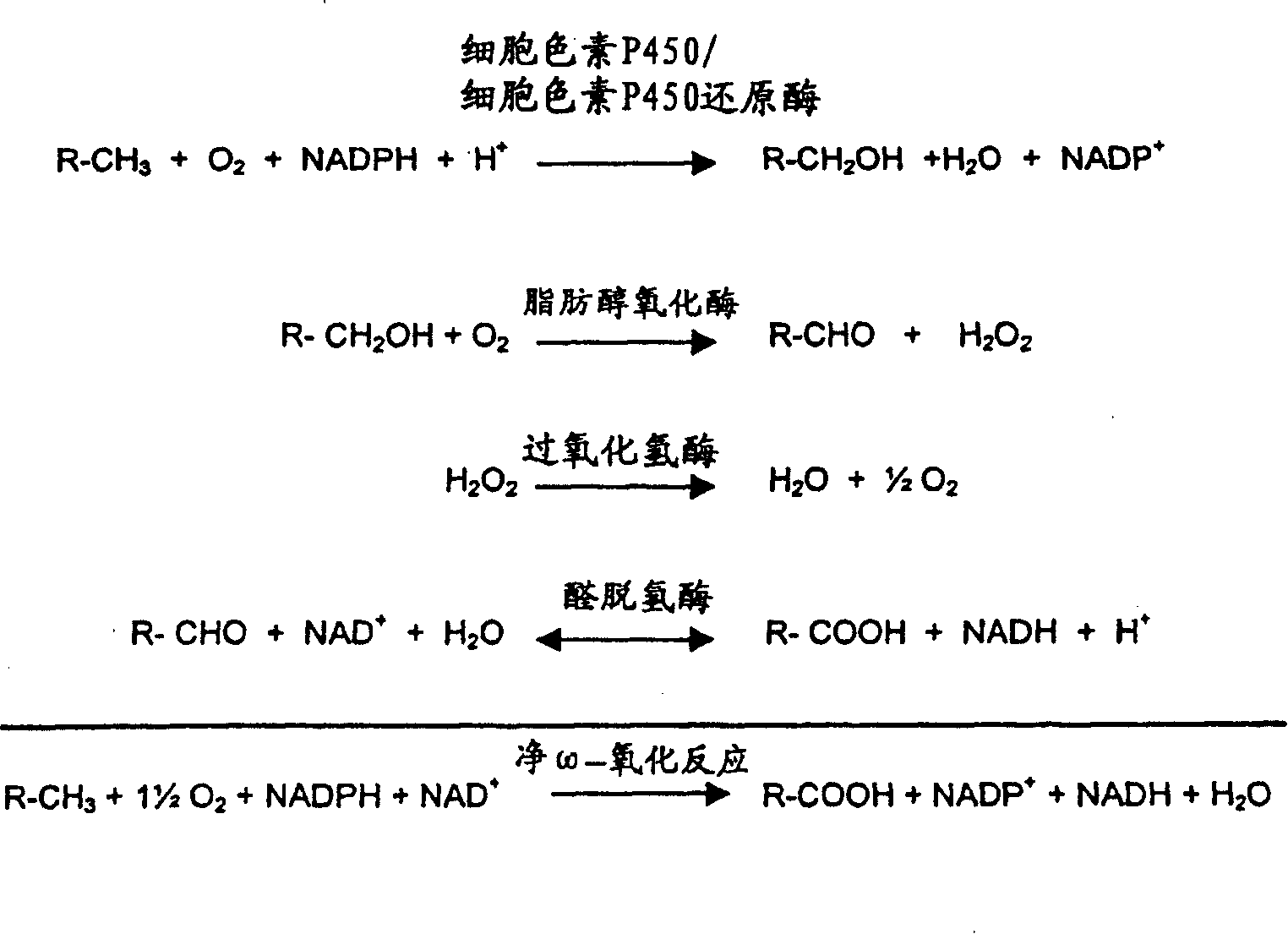
[0122] Operational and stoichiometric parameters for pure co-substrate oxidations
[0123] This example relates to the determination of the stoichiometric coefficient (Xc = 1 ) of Reaction 2 for the oxidation of a pure co-substrate feed. These coefficients are then used directly in the monitoring and control formulas of the present invention.
[0124] Reaction 2 represents a series of net biochemical reactions by which energy is produced from co-substrates via biocatalysts. It is not necessary to know the route in determining the stoichiometric coefficients for Reaction 2, since the stoichiometry is the same as for complete oxidation of the same co-substrate to carbon dioxide and water in the presence of oxygen. That is, chemical formula C x h y o z For the cosubstrate of , the oxidation stoichiometry is obtained as follows:
[0125] C x h y o z +(x+1 / 4y-1 / 2z)O 2 →xCO 2 +1 / 2yH 2 O Reaction 3.1
[0126] By checking with response 2 of this specification, then:
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com