Kernel-containing boracic acid group absorptive and its preparing method
A boric acid-based and adsorbent technology, which is applied in chemical instruments and methods, and other chemical processes, can solve the problems of long residence time of adsorbate, increased pressure drop of adsorbent bed, and increased power consumption in the separation process, etc. The effect of solid phase mass transfer resistance, increased adsorption mass transfer rate, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: the preparation of adsorbent of the present invention
[0025] The preparation method of adsorbent of the present invention comprises the following steps:
[0026] A) Preparation of polystyrene matrix
[0027] Make the toluene of 0.6g benzoyl peroxide as initiator and 100ml as porogen and dissolve in 99g styrene monomer and 45g divinylbenzene (as cross-linking agent, its content is 55%) mixed solution, add 700ml Dissolve 0.5% polyvinyl alcohol in deionized water, stir rapidly and heat up to carry out cross-linking copolymerization reaction. The temperature was raised to 80°C within 1 hour and maintained for 2 hours; then raised to 90°C and maintained for 2 hours; finally reacted at 96°C for another 5 hours. The spheres were filtered out, washed with hot water, the resulting mixture was filtered, solid-phase centrifuged, washed, and vacuum-dried at 65°C to obtain polystyrene matrix uniform pellets without adsorption activity, with a particle size distribu...
Embodiment 2
[0032] Embodiment two: the preparation of adsorbent of the present invention
[0033] The preparation method of adsorbent of the present invention comprises the following steps:
[0034] A) Preparation of Nucleated Matrix:
[0035] Make the toluene of 0.6g benzoyl peroxide as initiator and 100ml as porogen and dissolve in 99g styrene monomer and 45g divinylbenzene (as cross-linking agent, its content is 55%) mixed solution, add 700ml Dissolve 0.5% polyvinyl alcohol in deionized water, then add glass or quartz balls (50g) with a diameter of about 1.0-1.5mm and roughened surface, stir rapidly and heat up to carry out cross-linking copolymerization reaction. The temperature was raised to 80°C within 1 hour and maintained for 2 hours; then raised to 90°C and maintained for 2 hours; finally reacted at 96°C for 5 hours (by adjusting the reaction time, different thicknesses can be obtained on the surface of the inert sphere) Polystyrene, after subsequent treatment, can obtain the n...
Embodiment 3
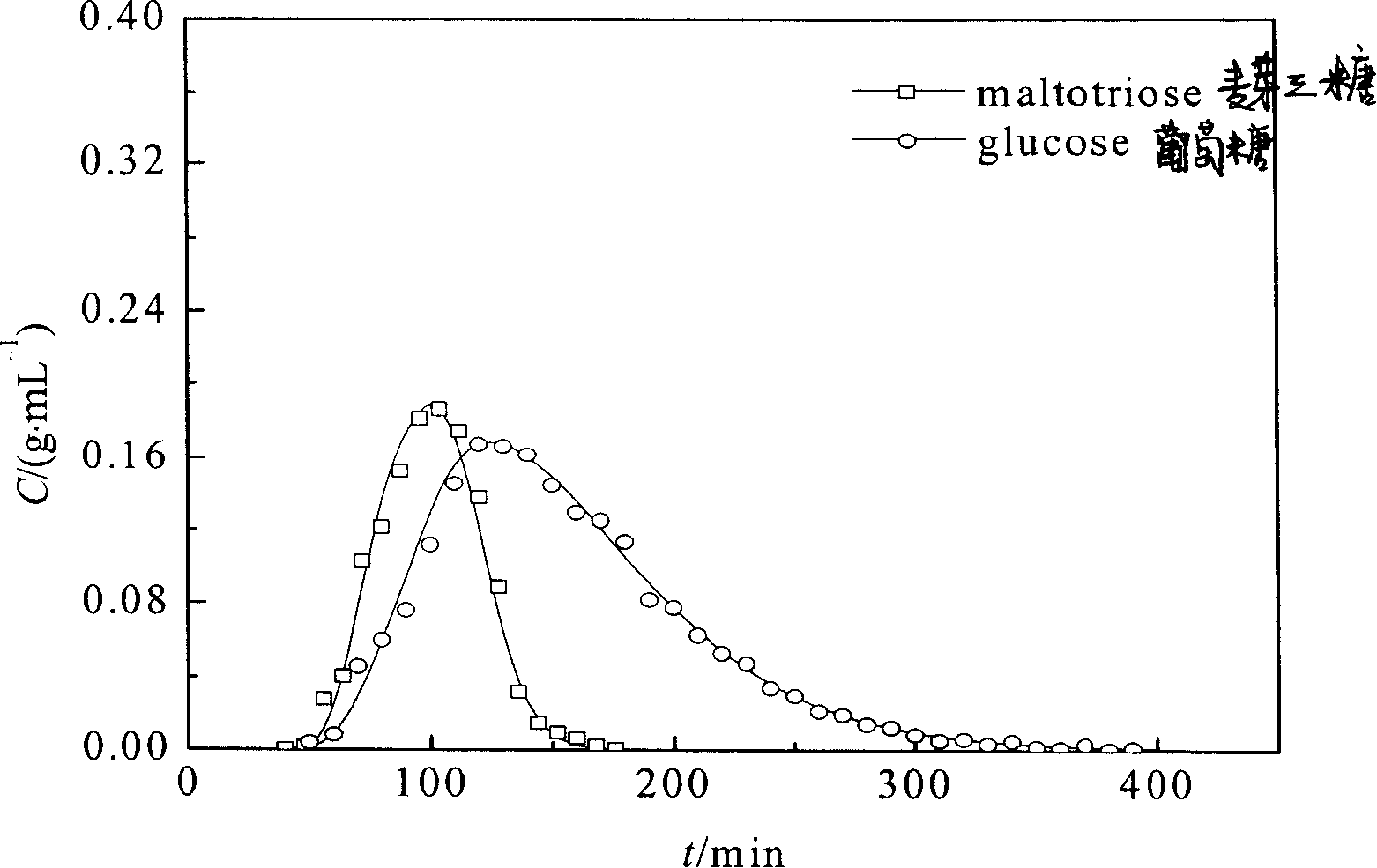
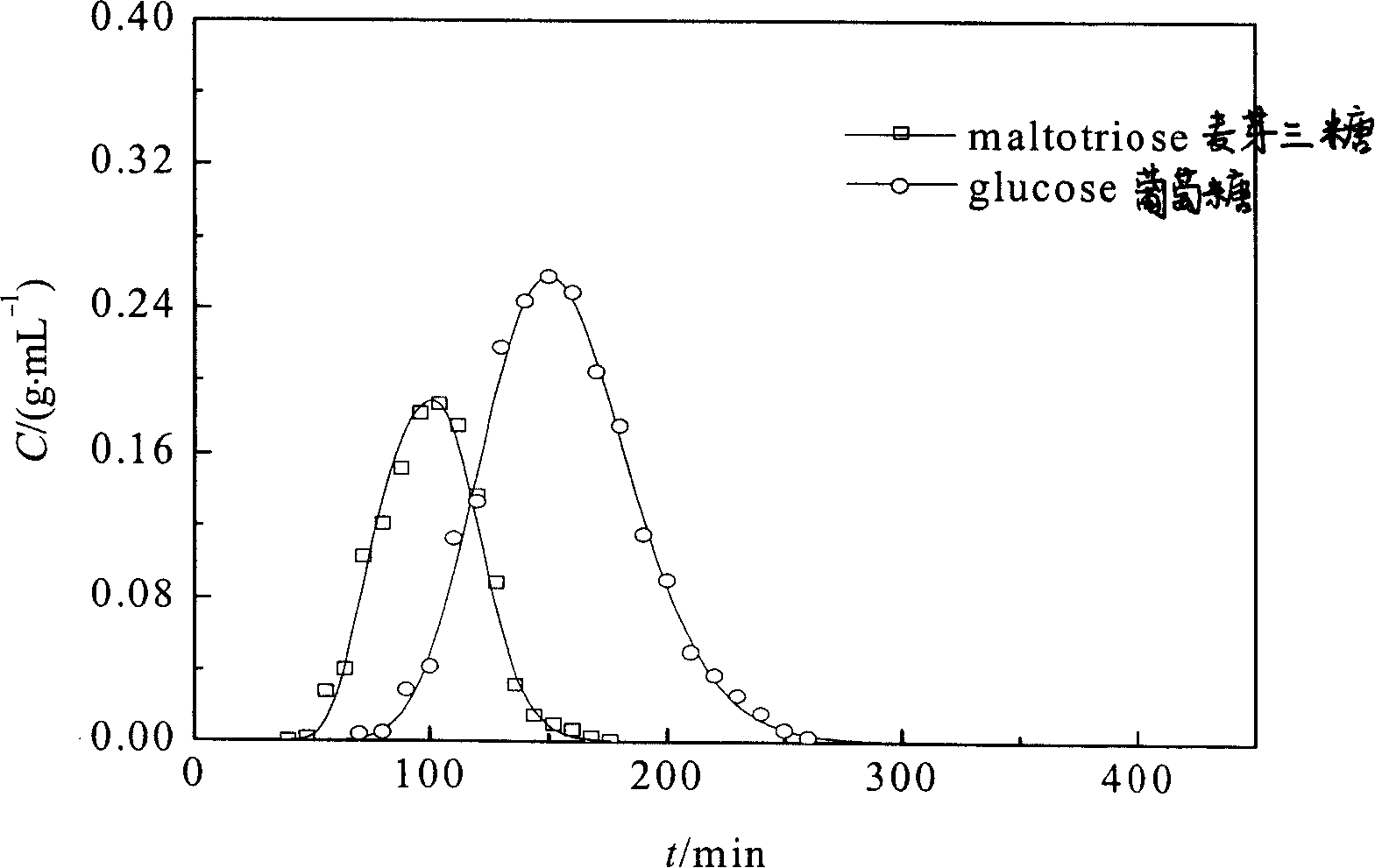
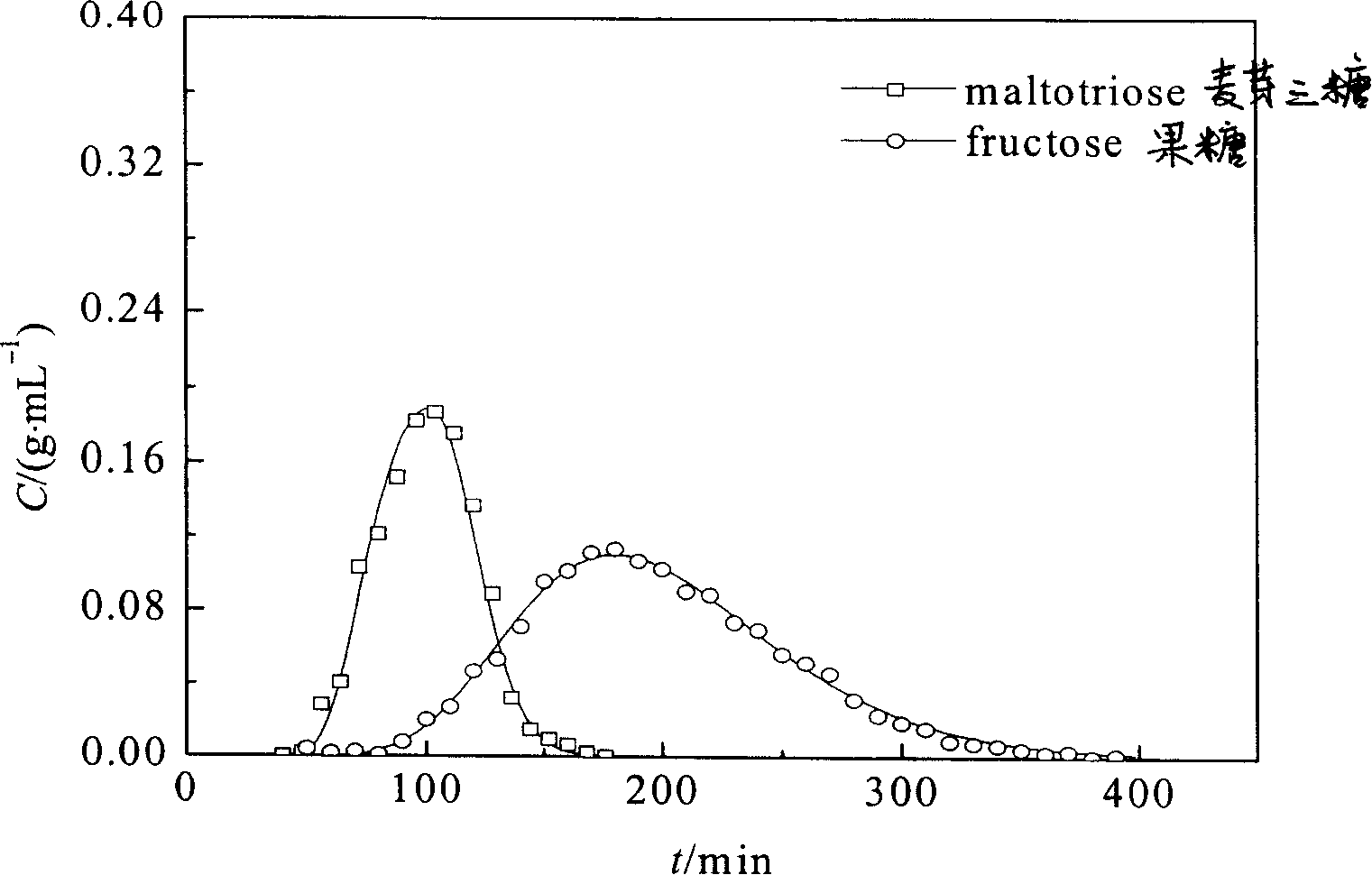
[0040] Example 3: Chromatographic separation experiment for preparation of glucose / maltotriose and fructose / maltotriose
[0041] (1) Experimental materials:
[0042] 1. Boric acid-based nucleated coordination adsorbent: prepared by the preparation method described in Example 1 and Example 2;
[0043] 2. Boric acid-based homogeneous coordination adsorbent: in the step B described in Example 1, the nitration time is long enough, and the polystyrene pellets are all nitrated, and the boric acid-based homogeneous coordination adsorbent is obtained through the subsequent treatment of step C ;
[0044] 3. Analytical pure glucose: Huakai Industrial Co., Ltd. (Shanghai);
[0045] 4. Fructose: Huakai Industrial Co., Ltd. (Shanghai);
[0046] 5. Maltotriose: Sigma (USA)
[0047] (2) Experimental process:
[0048] Take the above-mentioned boric acid-based nucleated coordination adsorbent or boric acid-based homogeneous phase, and wet-pack it into an adsorption column with an inner di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com