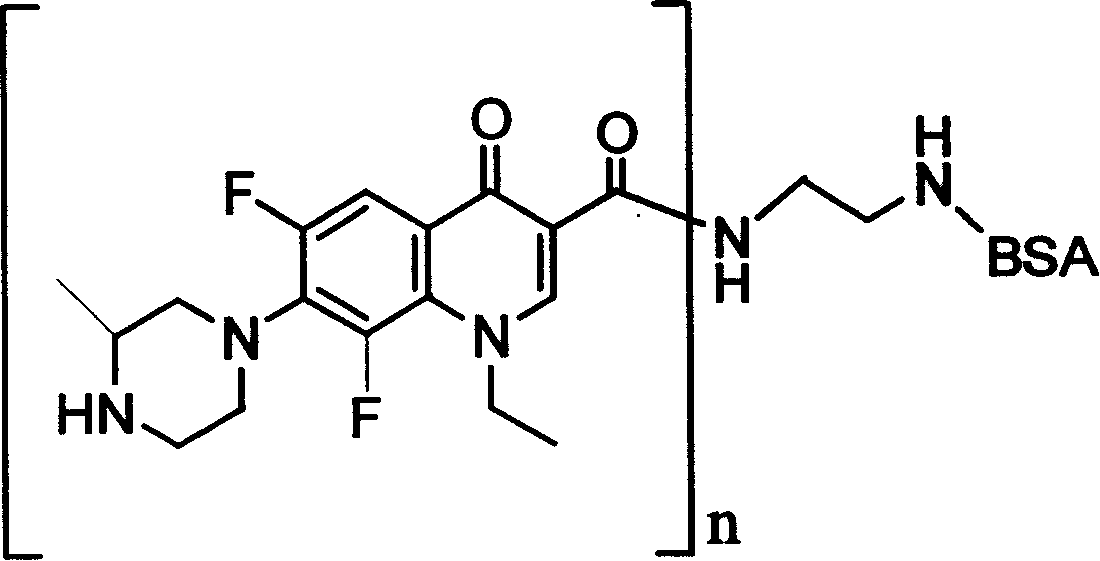
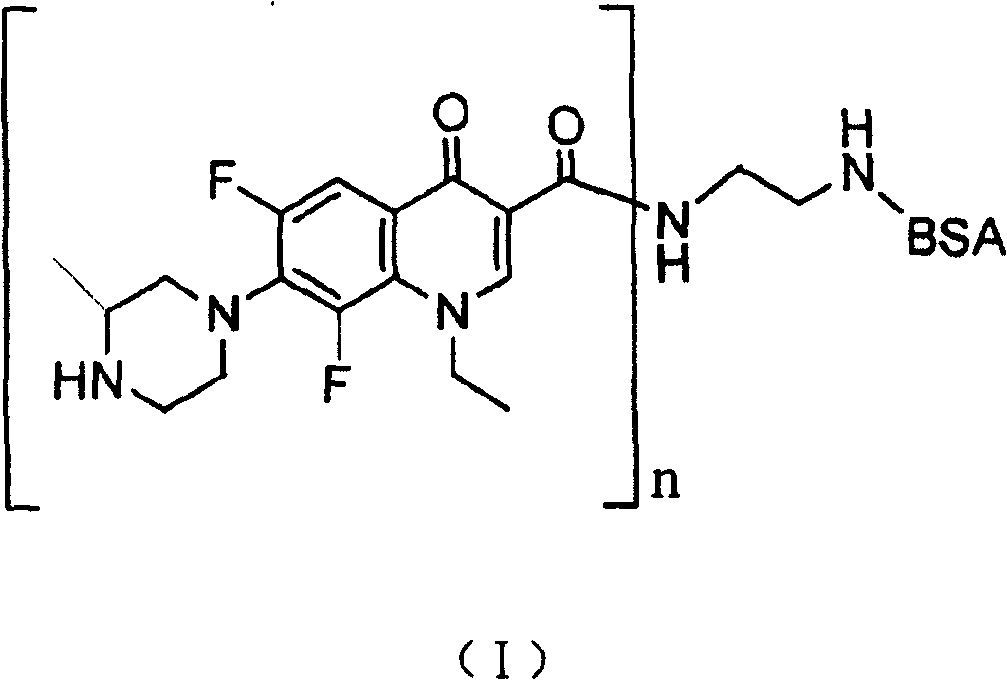
Lomefloxacin conjugate and its preparing method and use
A technology of lomefloxacin and conjugates, which is applied in the field of immunoassay of antibiotic drugs, can solve the problems of short shelf life, high price, and inability to meet the detection needs, and achieve the effect of saving inspection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of liquid A: Weigh 10.00 mg of lomefloxacin, 13.12 mg of hydroxysuccinimide, and 27.31 mg of EDC, dissolve them in 3 ml of dimethylformamide, and react to generate active intermediates of lomefloxacin and EDC. spare;
[0040](2) Preparation of cBSA: Dissolve 18.03mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.40 and a concentration of 0.01M at 0-4°C, adjust the pH to 7.40 with concentrated hydrochloric acid; weigh 1000.00 mg BSA (molecular weight: 68,000) and 57.51 mg EDC, then added to ethylenediamine solution, stirred and reacted at 20°C for 2 hours; The dialyzed solution was stirred and dialyzed for 70 hours, and then dialyzed with distilled water for 24 hours, and the dialysate was replaced every 6 hours; the dialyzed solution was centrifuged at 13,000 rpm at 0-4°C for 15 minutes, and the supernatant was taken; Clear liquid, obtain white powder solid cBSA, standby;
[0041] (3) Preparation of solution B: dissolve cBSA in ...
Embodiment 2
[0046] (1) Preparation of A: Weigh 10.00 mg of lomefloxacin, 16.40 mg of hydroxysuccinimide, and 54.63 mg of EDC, dissolve them in 4.6 ml of dimethylformamide, and react to generate an active intermediate of lomefloxacin and EDC, spare;
[0047] (2) Preparation of cBSA: Dissolve 18.03mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.40 and a concentration of 0.01M at 0-4°C, adjust the pH to 7.40 with concentrated hydrochloric acid; weigh 1000.00 mgBSA (molecular weight 68,000) and 57.51mg EDC, then added to the ethylenediamine solution, stirred and reacted at 25°C for 4 hours; the reaction solution of ethylenediamine and BSA was mixed with the above-mentioned phosphate buffer Stir and dialyze for 72 hours, then dialyze with distilled water for 30 hours, replace the dialysate every 6 hours; centrifuge the dialyzed solution at 13,000 rpm for 15 minutes at 0-4°C, take the supernatant; freeze-dry the supernatant Liquid, obtain white powder solid cBSA, stan...
Embodiment 3
[0053] (1) Preparation of liquid A: Weigh 10.00 mg of lomefloxacin, 26.24 mg of hydroxysuccinimide, and 81.95 mg of EDC, dissolve them in 10 ml of dimethylformamide, and react to generate active intermediates of lomefloxacin and EDC. spare;
[0054] (2) Preparation of cBSA: Dissolve 18.52mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.56 and a concentration of 0.02 M at 0-4°C, adjust the pH to 7.56 with concentrated hydrochloric acid; weigh 1000.00 mg BSA (molecular weight: 67,000) and 57.51 mg EDC, then added to ethylenediamine solution, stirred and reacted at 22°C for 3 hours; The dialyzed solution was stirred and dialyzed for 80 hours, and then dialyzed with distilled water for 20 hours, and the dialysate was replaced every 6 hours; the dialyzed solution was centrifuged at 13,000 rpm at 0-4°C for 15 minutes, and the supernatant was taken; freeze-dried Clear liquid, obtain white powder solid cBSA, standby;
[0055] (3) Preparation of solution B: d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com