Nasal cavity spraying inactivated influenza virus vaccine and its prepn process
A technology of influenza virus and vaccine, which is applied in the field of nasal spray type influenza virus inactivated vaccine and its preparation, and can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
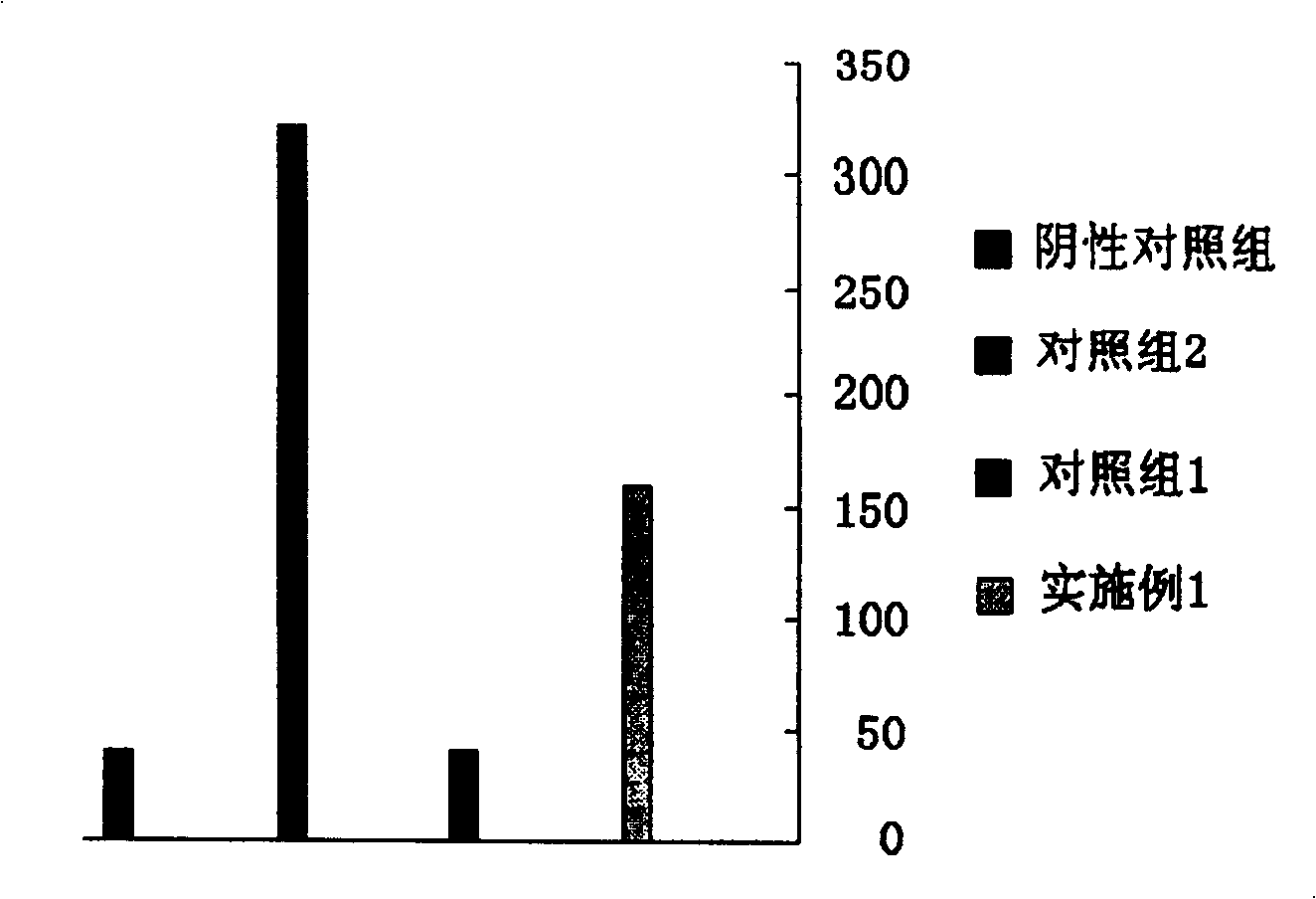
Image
Examples
Embodiment 1
[0082] Take 10,000 units of human recombinant interleukin 2, which was donated by Beijing Yuance Pharmaceutical Co., Ltd.; 1% chitosan was purchased from Sigma Company in the United States, with a molecular weight of 150kD and a deacetylation of more than 85%. The liquid is a phosphoric acid solution containing 1% benzyl alcohol, 1% mannitol, and 0.05% human serum albumin by weight, and its pH value is 7.0;
[0083] After the above three are mixed, mix with the trivalent split influenza virus hemagglutinin antigen (purchased from the Pasteur Institute) according to the volume ratio of 1:1 to prepare the trivalent split influenza virus inactivated vaccine nasal spray, each volume 100 μl for nasal immunization.
Embodiment 2
[0085] Apply the popular influenza virus strains recommended by WHO: H3N2 influenza A virus, H1N1 influenza A virus and influenza B virus. The three viruses were inoculated into the allantois of 9-11-day-old chicken embryos respectively, and after culturing at 37C for 48 hours, respectively Collect the allantoic fluid, use formalin to inactivate the virus, use SDS influenza virus lysing agent to lyse the virus particles, and then separate and purify respectively to obtain the lysed virus;
[0086] Will 5*10 5 A unit of human interferon α2b, 5g of starch was added to 500mL of phosphate buffer solution with a pH of 7.0, and the phosphate buffer solution contained 1g of human serum albumin, 1g of benzyl alcohol, and 5g of mannitol;
[0087] Get 500g split virus, add above-mentioned buffer solution, make trivalent split influenza virus inactivated vaccine nasal cavity spray, dose, be used for nasal cavity immunization.
Embodiment 3
[0089] Apply the influenza virus strains popular in the year recommended by WHO: H3N2 influenza A virus, H1N1 influenza A virus and influenza B virus. The three viruses were inoculated into the allantois of 9-11-day-old chicken embryos respectively. After culturing at 37C for 48 hours, Collect the allantoic fluid respectively, extract the virus hemagglutinin (HA) of three strains of viruses respectively;
[0090] take 10 4 Unit human recombinant interleukin 2 (gifted by Beijing Yuance Pharmaceutical Co., Ltd.), 10g of chitin was added to 500mL of phosphate buffer solution with a pH of 7.0, and the phosphate buffer solution contained 0.6g of human serum albumin, 5g of benzyl alcohol, 0.5 g mannitol;
[0091] Get 500g split virus, add above-mentioned buffer solution, make trivalent split influenza virus inactivated vaccine nasal cavity spray, dose, be used for nasal cavity immunization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com