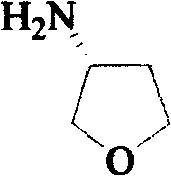
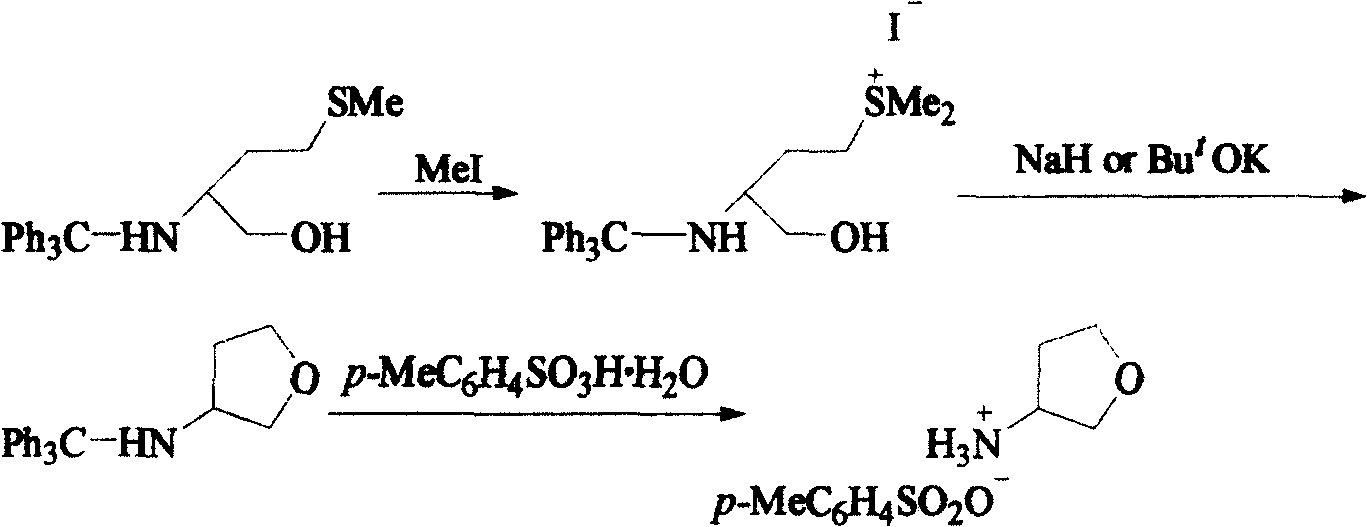
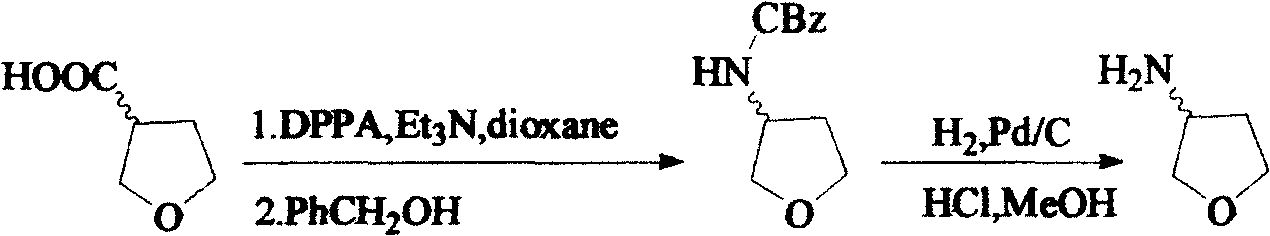Method for synthesizing (R)-3-amido tetrahydrofuran
A technology of aminotetrahydrofuran and hydroxytetrahydrofuran, which is applied in the field of synthesis of 3-aminotetrahydrofuran, can solve the problems of high toxicity of methyl iodide, large environmental pollution, and difficult availability of raw materials, and achieve the effects of low price, easy availability of raw materials, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Step 1. Preparation of (S)-3-tetrahydrofuran p-toluenesulfonate
[0022] (S)-3-Hydroxytetrahydrofuran (3g, 34mmol), p-toluenesulfonyl chloride (9.74g, 51mmol), triethylamine (7ml, 51mmol), DMAP (2.12g, 17mmol) were successively added to 50ml of dichloromethane, stirred, React at 60-70°C for 6-8 hours, TLC detects that the starting material disappears, and the reaction is stopped. Add 150ml of ethyl acetate, and wash with 100ml of 1N dilute hydrochloric acid, 100ml of water, and 100ml of saturated brine. After concentration under reduced pressure, 50 ml of water and 1 g of sodium hydroxide were added and stirred overnight. Extract with ethyl acetate 3 times, 30ml each time, combine the organic phases, and dry over anhydrous magnesium sulfate. Concentration under reduced pressure gave 7.26 g of (S)-3-tetrahydrofuran p-toluenesulfonate as a white solid, with a yield of 88%.
[0023] [ α ] D ...
Embodiment 2
[0033] Step 1. Preparation of (S)-3-tetrahydrofuran mesylate
[0034] (S)-3-Hydroxytetrahydrofuran (3g, 34mmol), methanesulfonyl chloride (5.84g, 51mmol), triethylamine (7ml, 50mmol), DMAP (2g, 16mmol) were added successively to 50ml of dichloromethane, stirred, 50~ React at 60°C for 6-8 hours, and stop the reaction when there is no raw material point detected by TLC. Add 150ml of ethyl acetate, and wash with 100ml of 1N dilute hydrochloric acid, 100ml of water, and 100ml of saturated brine. After concentration under reduced pressure, 50 ml of water and 1 g of sodium hydroxide were added and stirred overnight. Extract with ethyl acetate 3 times, 30ml each time, combine the organic phases, and dry over anhydrous magnesium sulfate. Concentration under reduced pressure gave 4.93 g of light yellow liquid with a yield of 87.1%.
[0035] Step 2. Preparation of (R)-3-aminotetrahydrofuran is the same as in Example 1.
Embodiment 3
[0037] Step 1. Preparation of (S)-3-tetrahydrofuran benzenesulfonate
[0038] (S)-3-Hydroxytetrahydrofuran (3g, 34mmol), benzenesulfonyl chloride (9g, 51mmol), triethylamine (7ml, 50mmol), DMAP (2g, 16mmol) were successively added to 50ml of chloroform, stirred, 60-70 ℃ reaction, 6-8 hours, TLC detection no raw material point, stop the reaction. Add 150ml of ethyl acetate, and wash with 100ml of 1N dilute hydrochloric acid, 100ml of water, and 100ml of saturated brine. After concentration under reduced pressure, 50 ml of water and 1 g of sodium hydroxide were added and stirred overnight. Extract with ethyl acetate 3 times, 30ml each time, combine the organic phases, and dry over anhydrous magnesium sulfate. Concentration under reduced pressure afforded 6.71 g of white solid with a yield of 86.3%.
[0039] Step 2. Preparation of (R)-3-aminotetrahydrofuran is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com