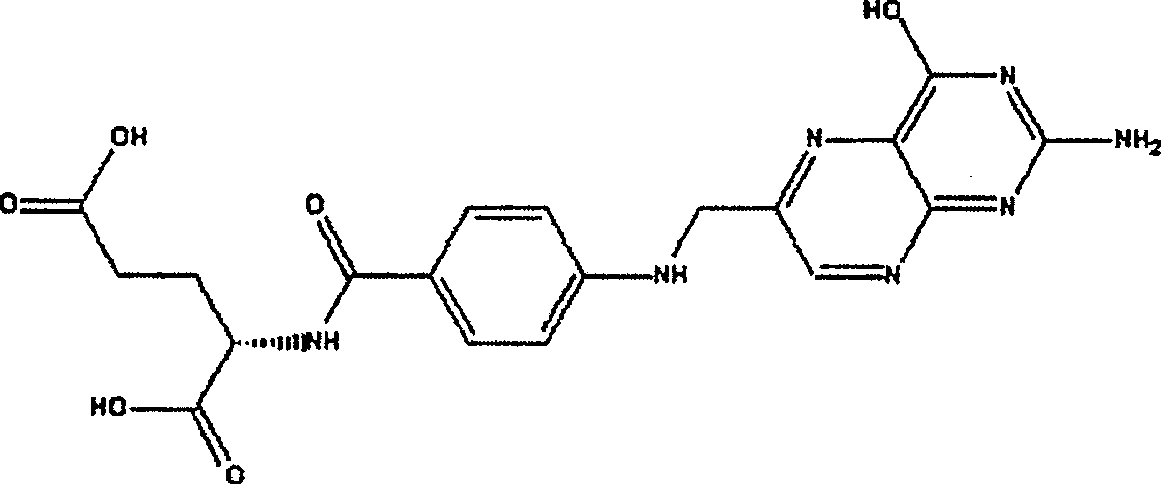
Folic acid freeze-dried injection and preparation thereof
A freeze-dried powder for injection and a technology for powder for injection are applied in the field of folic acid freeze-dried powder for injection and its preparation, and can solve the problems of difficulty in making folic acid powder for injection, poor water solubility, turbidity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
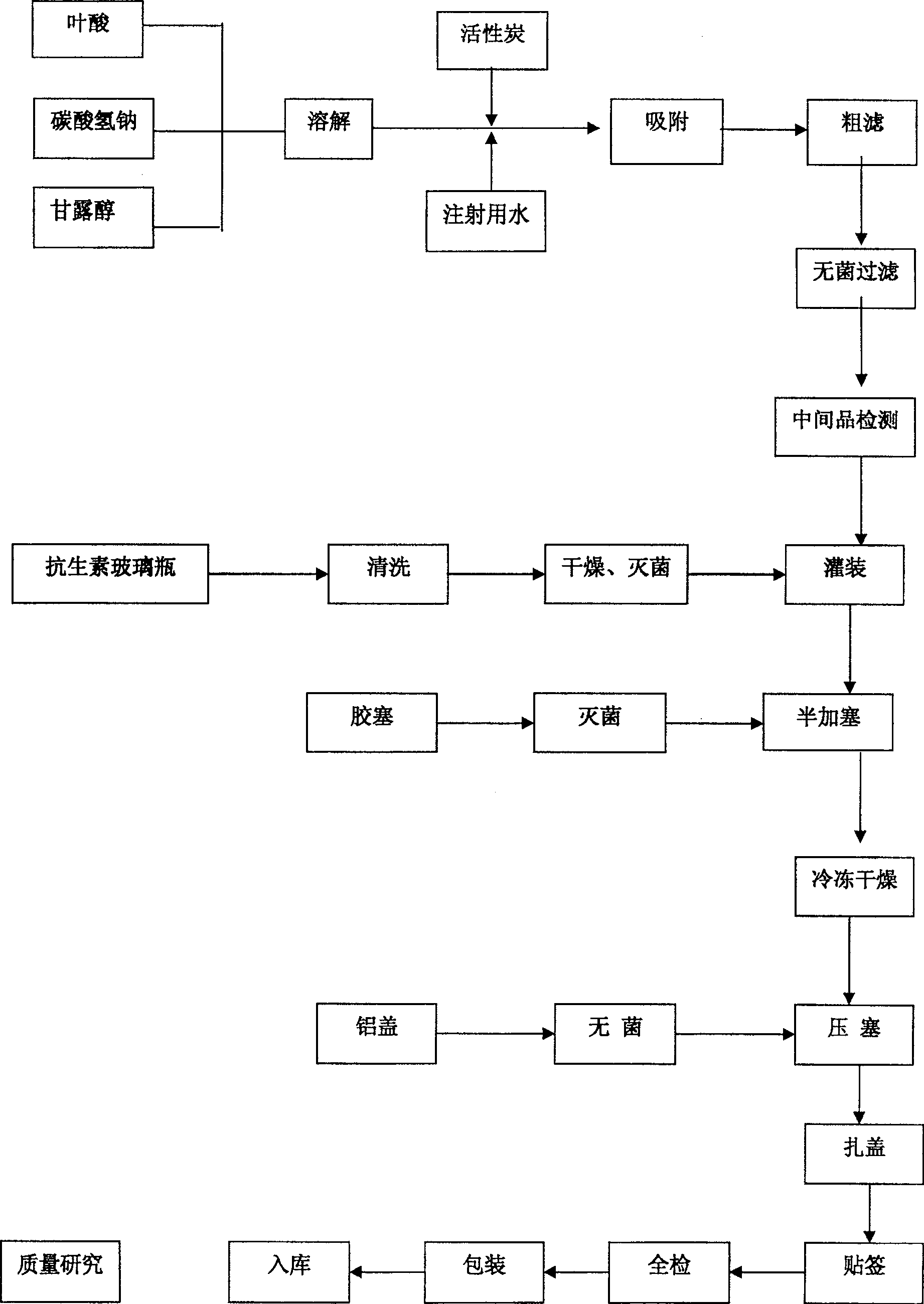
[0057] Folic acid 15g, mannitol 100g, mix appropriate amount of water for injection, folic acid and mannitol, and stir until it becomes a paste; slowly add 4% sodium bicarbonate solution to dissolve it all, and make the pH value at 6.5, then stir for 20 minutes After the pH remains unchanged, add water for injection to 2000ml, then coarsely filter with activated carbon with a concentration of 0.005mg / ml, and then pass through 1.0um, 0.45um, 0.22um microporous membranes to sterilize and filter, and then filter into the sterile room. Measure the pH value and folic acid content of the solution. After passing the test, fill it, press half the plug, put it into a freeze-drying box that has been cooled to -50°C, and pre-freeze for 100 minutes.
[0058] After the drug is frozen, start the vacuum machine to evacuate to 10Pa, turn off the freezer, heat the drug to make the temperature of the frozen product rise to -25°C; the time is 20 hours and 20 minutes, then gradually heat the drug ...
Embodiment 2
[0060] Folic acid 30g, mannitol 100g, mix appropriate amount of water for injection, folic acid and mannitol, and stir until it becomes a paste; slowly add 4% sodium bicarbonate solution to dissolve it all, and make the pH value at 8.5, then stir for 20 minutes After the pH remains unchanged, add water for injection to 2000ml, then coarsely filter with activated carbon with a concentration of 0.005mg / ml, and then pass through 1.0um, 0.45um, 0.22um microporous membranes to sterilize and filter, and then filter into the sterile room. Measure the pH value and folic acid content of the solution. After passing the test, fill it, press half the plug, put it into a freeze-drying box that has been cooled to -50°C, and pre-freeze for 100 minutes.
[0061] After the drug is frozen, start the vacuum machine to evacuate to 10Pa, turn off the freezer, heat the drug to make the temperature of the frozen product rise to -25°C; the time is 20 hours and 20 minutes, then gradually heat the drug ...
Embodiment 3
[0063] Folic acid 40g, mannitol 200g, mix appropriate amount of water for injection, folic acid and mannitol, and stir until it becomes a paste; slowly add 4% sodium bicarbonate solution to dissolve it all, and make the pH value at 7.0, then stir for 20 minutes After the pH remains unchanged, add water for injection to 2000ml, then use activated carbon with a concentration of 0.005mg / ml for coarse filtration, then; room, measure the pH value and folic acid content of the solution, after passing the test, fill it, press half the plug, put it into a freeze-drying box that has been cooled to -50°C, and pre-freeze for 100 minutes.
[0064] After the drug is frozen, start the vacuum machine to evacuate to 10Pa, turn off the freezer, heat the drug to make the temperature of the frozen product rise to -25°C; the time is 20 hours and 20 minutes, then gradually heat the drug to 0°C, and keep it vacuum-dried for 10 hours. Continue to heat up to 25°C and vacuum-dry for 3 hours, press the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com