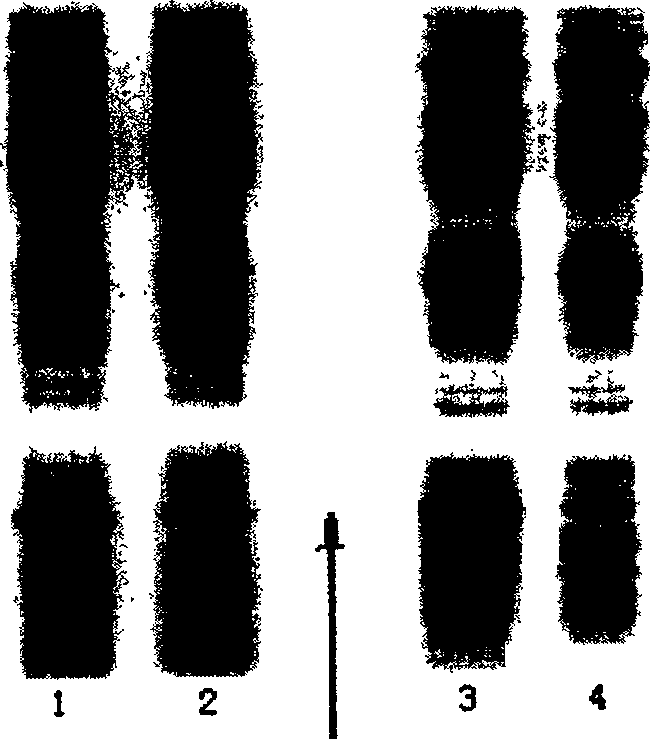Expression of soluble TRAIL protein
A soluble protein technology, applied in the field of bioactive protein expression, can solve the problems of high inclusion body content and low soluble TRAIL protein content, and achieve the effect of increasing expression and reducing the ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0052] Example 2. Using 38°C as the induction temperature to induce the expression of TRAIL protein
[0053] After the recombinant Escherichia coli strain C600-TRAIL was activated in 30mL LB medium at 30°C, it was inoculated into 30mL LB medium at 1% inoculum size and cultured at 30°C. Before inoculation, ampicillin should be added to a concentration of 100μg / mL. Grow to OD 600 0.60, the shaker flask was placed in a shaker at 38°C for 8 hours, and the rotation speed was 200rpm during the growth phase and the induction phase.
[0054] Among them, the composition of LB medium is 10g / L peptone, 5g / L yeast powder, 5g / L sodium chloride, and the pH is adjusted to 7.0.
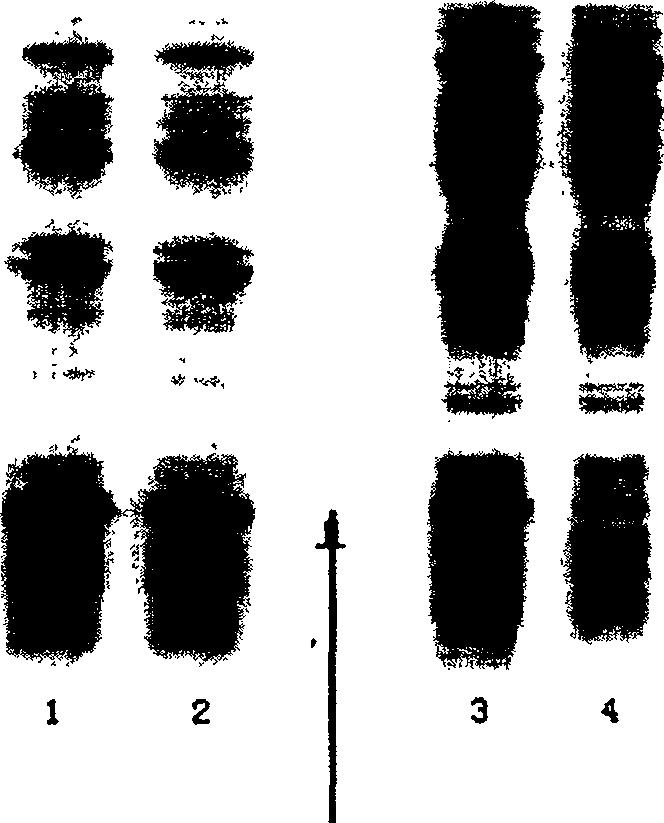
[0055] Treat the thalli in the same manner as in Example 1, carry out SDS-PAGE electrophoresis to the supernatant and the whole bacterium lysate, and scan the electrophoretic pattern to quantify the target product, see figure 1 . Compared with the usual induction expression at 42°C, when 38°C is used as the induct...
Embodiment 3、38
[0056] Example 3, 38 degree induction, adding 1mmol / L zinc ion improves the soluble expression of recombinant TRAIL protein
[0057] After the recombinant Escherichia coli strain C600-TRAIL was activated in 30mL LB medium at 30°C, it was inoculated into 30mL LB medium at an inoculum size of 1%, and cultured at 30°C. Before inoculation, ampicillin was required to be added to a concentration of 100 μg / mL. Grow to OD 600 0.585, divalent zinc ions were added under aseptic conditions to a concentration of 1 mmol / L, and the shake flask was placed in a shaker at 38°C for induction. The speed of the growth phase and the induction phase were both 200 rpm, and the induction was performed for 8 hours.
[0058] Among them, the composition of LB medium is 10g / L peptone, 5g / L yeast powder, 5g / L sodium chloride, and the pH is adjusted to 7.0. Using ZnSO 4 aqueous solution.
[0059] Treat the thalli in the same manner as in Example 1, carry out SDS-PAGE electrophoresis to the supernatant ...
Embodiment 5、38
[0064] Example 5, 38-degree induction, adding tetracycline that inhibits protein synthesis improves the soluble expression of recombinant TRAIL protein
[0065] After the recombinant Escherichia coli strain C600-TRAIL was activated in 30mL LB medium at 30°C, it was inoculated into two parts of 30mL LB medium according to the inoculum size of 1%, and cultured at 30°C. Before inoculation, it was necessary to add ampicillin to The concentration is 100 μg / mL. Two cultures were grown separately to OD 600 They were 0.530 and 0.562, respectively, and tetracycline was added under sterile conditions to a concentration of 4 μg / mL and 8 μg / mL. The shake flask was placed in a shaker at 38° C. for induction, and the rotation speed of both the growth phase and the induction phase was 200 rpm, and the induction was performed for 8 hours.
[0066] Among them, the composition of LB medium is 10g / L peptone, 5g / L yeast powder, 5g / L sodium chloride, and the pH is adjusted to 7.0. Antibiotics a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com