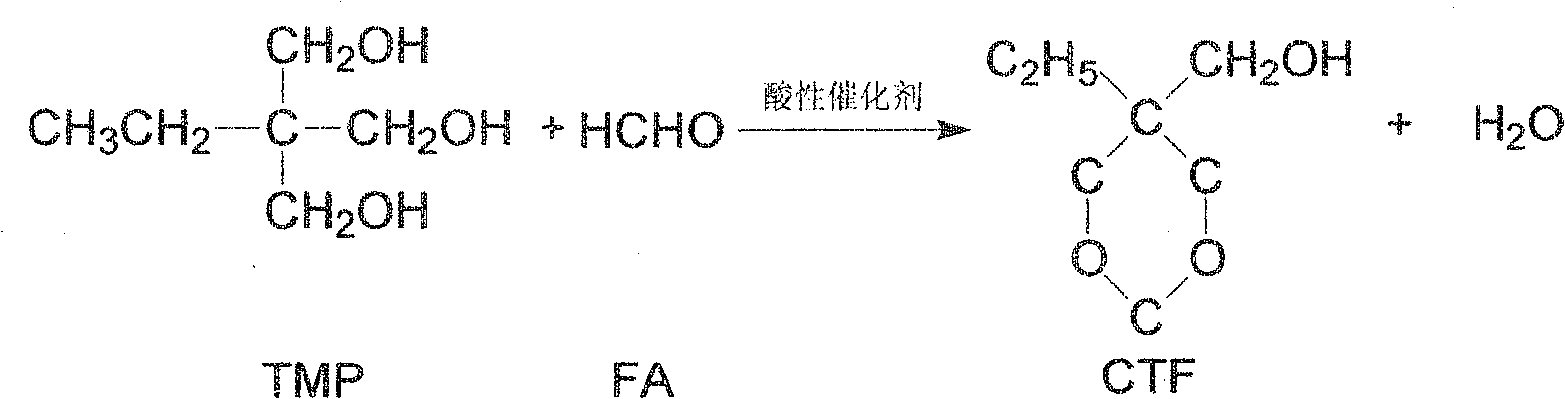
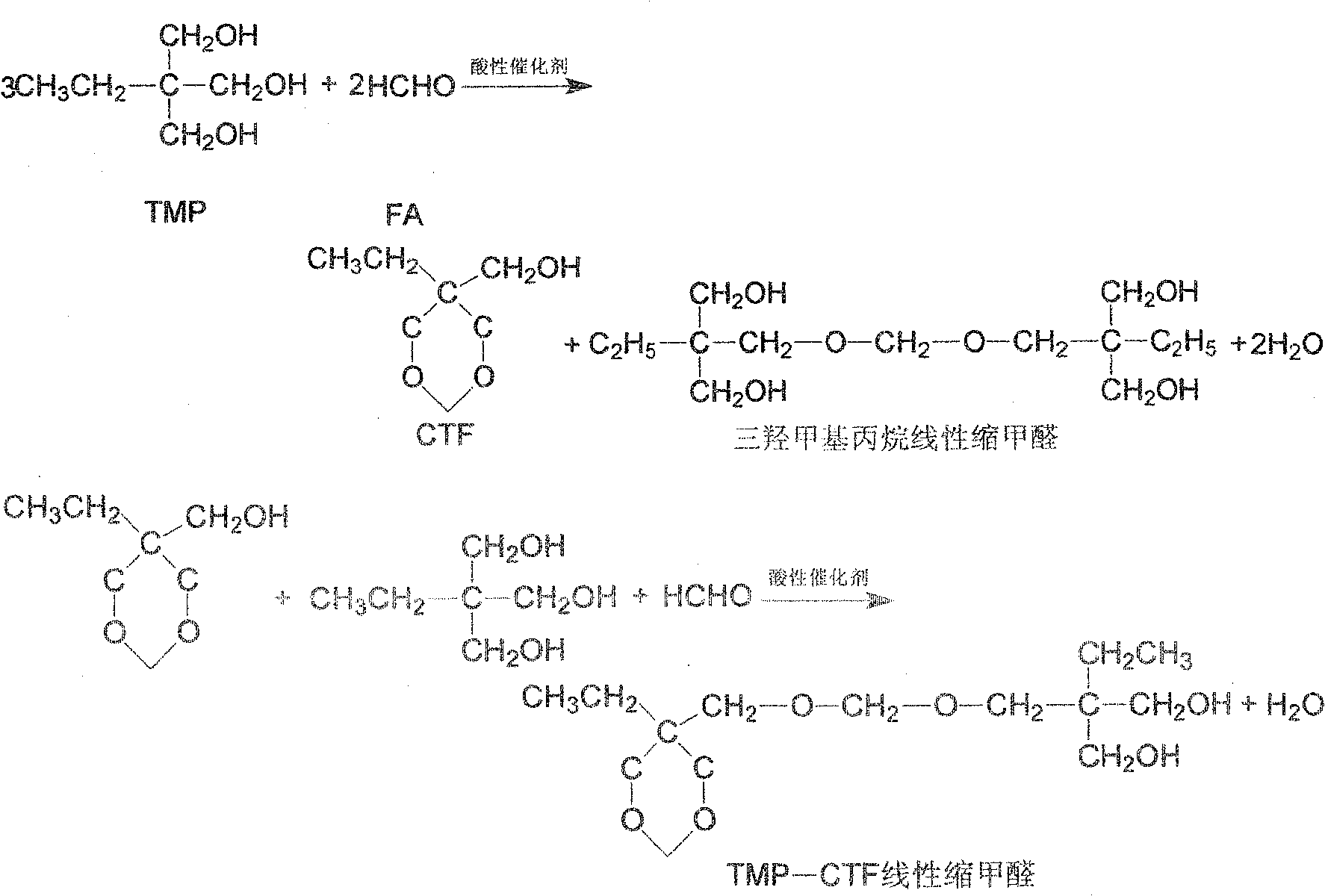
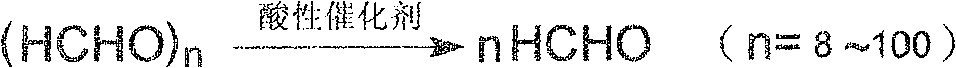Process of synthesizing trihydroxy methyl propane acetal
A technology of trimethylolpropane and formal, which is applied in the direction of organic chemistry, can solve problems such as unfavorable product yield, poor reaction effect, and reduced economy, and achieve process simplification, increase production efficiency, and reduce energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 201g of trimethylolpropane and 48g of paraformaldehyde into the reaction distillation device, then pass in nitrogen gas by bubbling and dispersing at the bottom and raise the temperature to 125°C, add 2g of sodium bisulfate, and carry out the aldehyde reaction under normal pressure. Water is discharged from the reaction system from the top of the tower. When no reaction water is discharged from the top of the tower, it is reacted for 3 hours at an absolute pressure of 2.67KPa and a reaction temperature of 145°C. Evaporated and dried. Based on the added amount of trimethylolpropane, the CTF yield is 96.4%.
Embodiment 2
[0032] According to the same procedure of embodiment 1, except adding 0.02g sodium bisulfate, all the other conditions remain unchanged. Based on the added amount of trimethylolpropane, the CTF yield is 91.5%.
Embodiment 3
[0034] According to the same procedure of embodiment 1, except adding 8g sodium bisulfate, all the other conditions remain unchanged. Based on the added amount of trimethylolpropane, the CTF yield is 94.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com