Synthesis process of 18F labeled positive electron radioactive tracer with ionic liquid as phase transfer catalyst
A technology of phase transfer catalyst and radioactive tracer, which is applied in radioactive carriers, organic chemical methods, chemical instruments and methods, etc. It can solve the problems of inability to have water components, insoluble polypeptides or proteins, etc., and achieve good specificity Sexual affinity, significant economic and social benefits, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] [ 18 F]FB-HYNIC-E-[c[RGDyK] 2 ] The synthesis steps:
[0076] 1. Will be transmitted from the medical cyclotron 18 F ions and water pass to the receiving bottle (1-4Ci);
[0077] 2. Will 18 F ions and water pass to the anion exchange column QMA;
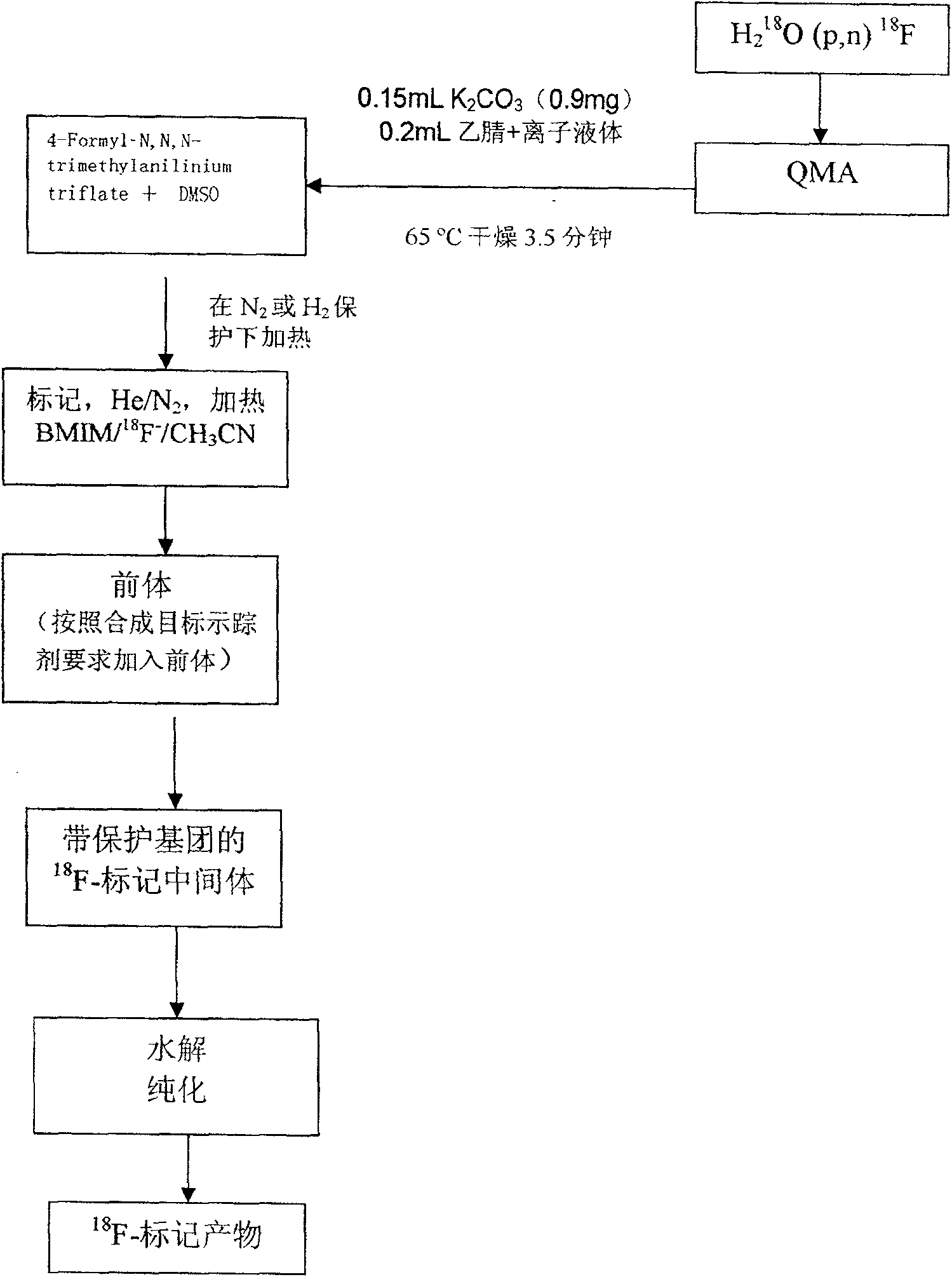
[0078] 3. Using 0.15mL K 2 CO 3 (0.9mg)+0.2mL acetonitrile+0.3mL ionic liquid ([BMIm][OTf]) was eluted from the QMA column 18 F ion and sent to the reaction flask, 4-Formy]-N, N, N-trimethylanilinium triflate 5mg dissolved in 0.5mL DMSO mixture was added to the reaction flask; under the protection of nitrogen or helium, heated at 65 degrees for 3.5 minutes, 90 Heating at 100°C for 10 minutes;
[0079] 4. The precursor (HYNIC-E-[c[RGDyK] 2 ], 2 mg) dissolved in Na 2 HPO 4 Buffer solution (pH8.5), mix thoroughly before adding to the reaction bottle; heat at 65°C for 25 minutes under the protection of nitrogen or helium;
[0080] 5. Cool the temperature to 35°C, then add HCl (1N, 0.5ml) solution into the reaction flas...
Embodiment 2
[0086] [ 18 F] Synthetic steps of FB-RGD:
[0087] 1. Will be transmitted from the medical cyclotron 18 F ions and water pass to the receiving bottle (1-4Ci);
[0088] 2. Will 18 F ions and water pass to the anion exchange column QMA;
[0089] 3. Using 0.15mL K 2 CO 3 (0.9mg)+0.2mL acetonitrile+0.3mL ionic liquid ([BMIm][OTf]) was eluted from the QMA column 18 F ion and sent to the reaction bottle, the mixture of 4-Formyl-N, N, N-trimethylanilinium triflate 5mg dissolved in 0.5mL DMSO was added to the reaction bottle; under the protection of nitrogen or helium, heated at 65 degrees for 3.5 minutes, 90 degrees Heating at low temperature for 10 minutes;
[0090] 4. Dissolve the precursor (c[RGDyK], 2 mg) in Na 2 HPO 4 Buffer solution (pH8.5), mix thoroughly before adding to the reaction bottle; heat at 65°C for 25 minutes under the protection of nitrogen or helium;
[0091] 5. Cool the temperature to 33°C, then add HCl (1N, 0.5ml) solution into the reaction flask;
[...
Embodiment 3
[0097] [ 18 F] Synthetic steps of Galacto-RGD:
[0098] 1. Will be transmitted from the medical cyclotron 18 F ions and water pass to the receiving bottle (1-4Ci);
[0099] 2. Will 18 F ions and water pass to the anion exchange column QMA;
[0100] 3. Using 0.15mL K 2 CO 3 (0.9mg)+0.2mL acetonitrile+0.3mL ionic liquid ([BMIm][OTf]) was eluted from the QMA column 18 F ion and sent to the reaction bottle, the mixture of 4-Formyl-N, N, N-trimethylanilinium triflate 5mg dissolved in 0.5mL DMSO was added to the reaction bottle; under the protection of nitrogen or helium, heated at 65 degrees for 3.5 minutes, 90 degrees Heating at low temperature for 10 minutes;
[0101] 4. Dissolve the precursor (cyclo(-Arg-Gly-Asp-D-Phe-Lys(SAA), 2mg) in 0.5mL DMSO and mix thoroughly before adding to the reaction flask, and then add 1M 0.5mLNaOH; Or heated at 70 degrees for 15 minutes under the protection of helium;
[0102] 5. Cool the temperature to 33°C, then add HCl (1N, 0.5ml) soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com