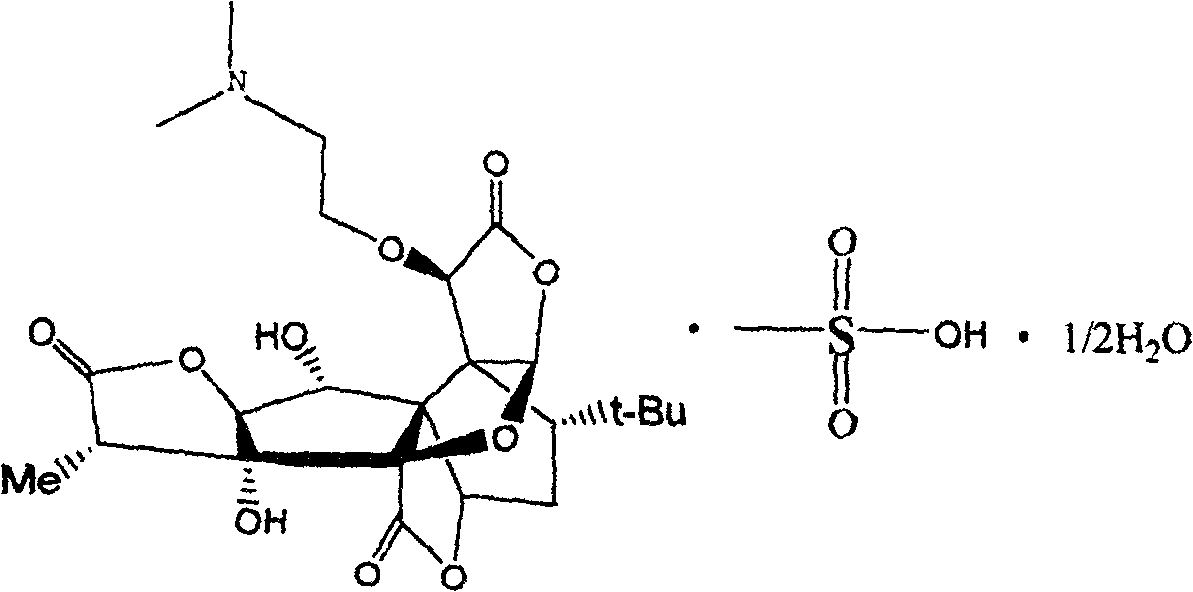
Leucinocaine 10-O-(dimethylaminoethyl)ginkgolide B semihydrate crystal and preparation method thereof
A technology of dimethylaminoethyl and ginkgolide, which is applied in the field of medicine and chemical industry, can solve the problems of dangerous operation, cumbersome synthesis steps, and difficult production, and achieve the effect of easy operation, simple preparation steps, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of 10-O-(dimethylaminoethyl) ginkgolide B
[0016] 250g (0.59mol) of ginkgolide B was dissolved in 8.6L of acetonitrile, followed by adding 130g of dimethylaminoethyl chloride hydrochloride, 800g of potassium carbonate, 100g of potassium iodide, and 20g of tetrabutylammonium bromide, and heated to reflux for 1 hour. The reaction is almost complete. Cool, filter, and concentrate the filtrate under reduced pressure to obtain a crude solid. The crude product was purified, recrystallized from methanol, and dried to obtain 117 g of solid (yield 40%).
Embodiment 2
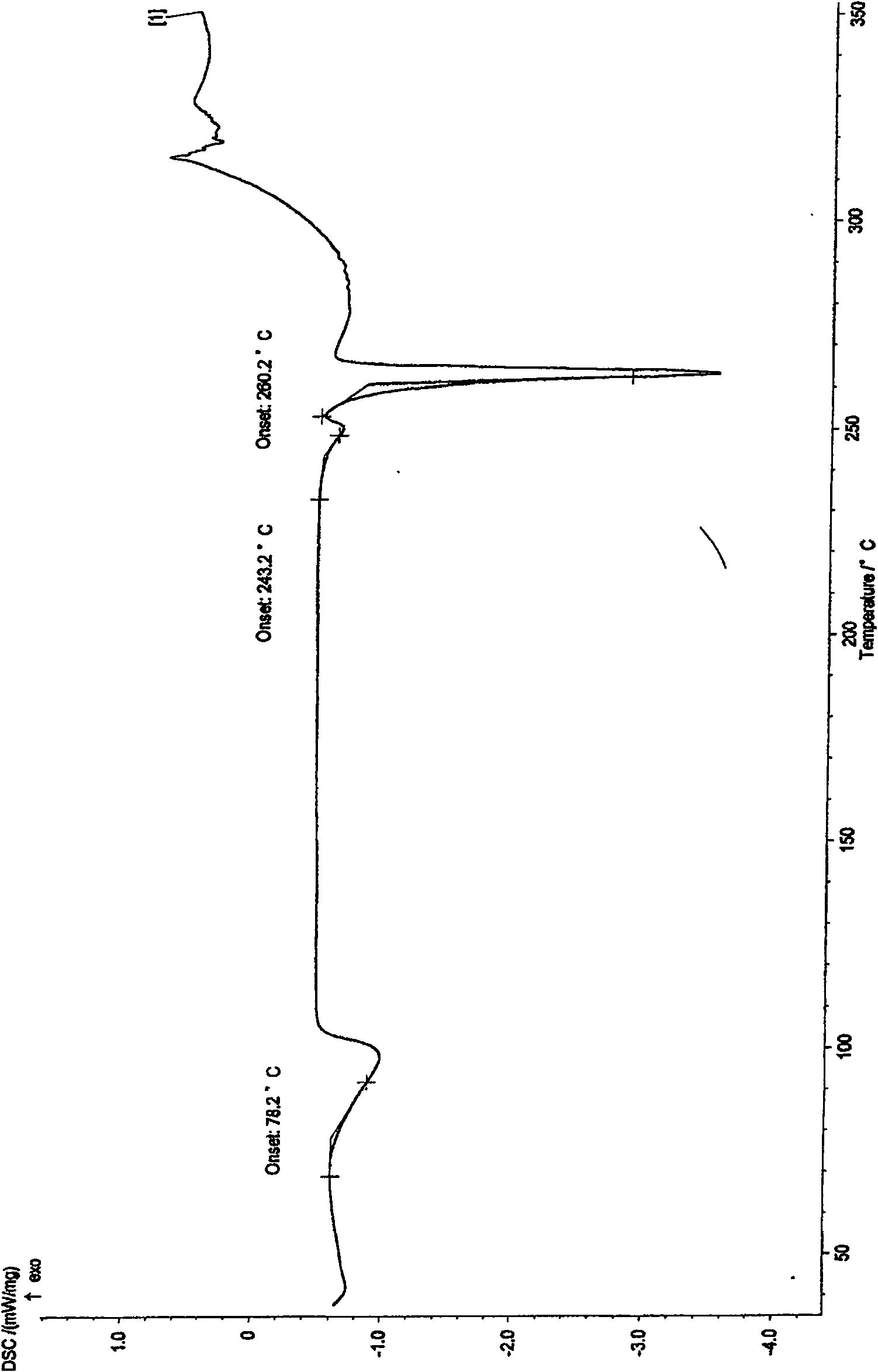
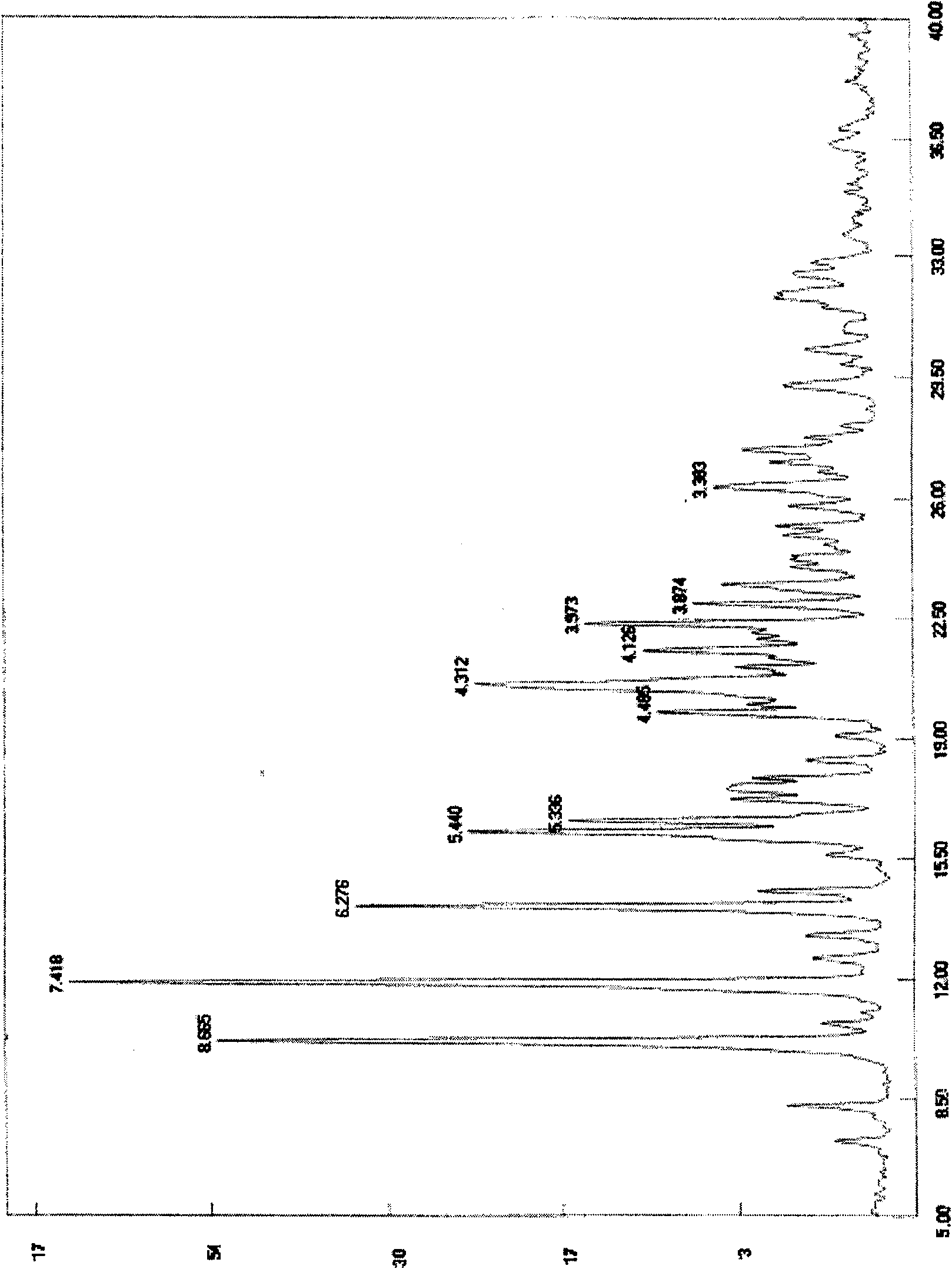
[0017] Example 2 Preparation of methanesulfonic acid 10-O-(dimethylaminoethyl) ginkgolide B hemihydrate crystallization 10-O-(dimethylaminoethyl) ginkgolide B 10g, methanol 100ml, stirring, Add 41g of 4.8% methanol solution of methanesulfonic acid dropwise, heat slightly, add a small amount of activated carbon after fully dissolved, reflux, filter while hot, cool and crystallize, filter, wash with methanol, add the obtained solid to 100ml of 90% methanol, heat Dissolve, then cool to crystallize, filter, and wash with 90% methanol to obtain methanesulfonic acid 10-O-(dimethylaminoethyl) ginkgolide B hemihydrate crystals.
Embodiment 3
[0018] The preparation of embodiment 3 methanesulfonic acid 10-O-(dimethylaminoethyl) ginkgolide B hemihydrate crystals
[0019] 10g of 10-O-(dimethylaminoethyl) ginkgolide B, 100ml of ethanol, stir, add dropwise 41g of 4.8% methanesulfonic acid ethanol solution, heat slightly, add a small amount of activated carbon after fully dissolved, reflux, while hot Filtration, crystallization by cooling, filtration, washing with ethanol, the resulting solid was added to 100ml of 90% ethanol, heated to dissolve, then cooled to crystallize, filtered, washed with 90% ethanol to obtain methanesulfonic acid 10-O-(dimethylaminoethyl Base) Ginkgolide B hemihydrate crystallization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com