Method for synthesizing (R)-toliprolol
A kind of synthesis method, the technology of molar ratio, is applied in the synthesis field of -tolirolol, can solve the problems such as not being suitable for industrialized application, reagent price is expensive, needs, achieves good industrialized application prospect, optical purity and yield are high, reduces by-product effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
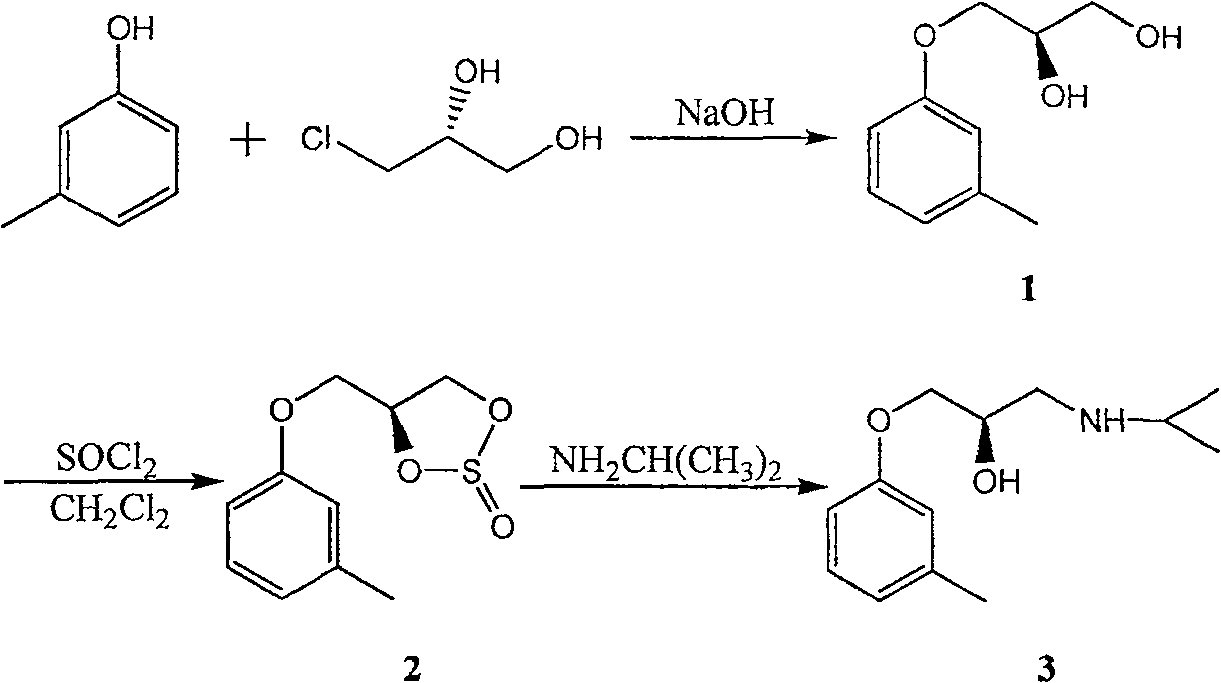
[0019] Embodiment 1: Preparation of (R)-Toliolol (Compound 3)
[0020] (1) Preparation of compound 1 [(R)-3-m-tolyloxy-1,2-propanediol]
[0021] Dissolve m-cresol (10.8g, 0.1mol) in 120mL absolute ethanol, add sodium hydroxide (5.1g, 0.13mol) in batches at room temperature, then add phase transfer catalyst tetrabutylammonium bromide (0.16g, 0.5mmol), drop (R)-3-chloro-1,2-propanediol (14.4g, 0.13mol), dropwise, 70 ~ 73 ° C reaction for 5 hours, the reaction is complete, filtered while hot, and the filtrate was concentrated under reduced pressure, Petroleum ether was recrystallized to obtain 14.8 g of white solid (R)-3-m-tolyloxy-1,2-propanediol with a yield of 81.2%. The experimental data are as follows:
[0022] mp 60-62°C, [α] D 20 = -9.5 (c 1.0, EtOH); 1 HNMR (400MHz, CDCl 3 )δ (ppm): 2.33 (s, 3H, CH 3 ), 3.71-3.84 (m, 2H, CH 2 OH), 4.04-4.06 (m, 2H, ArO CH 2 ), 4.11-4.13 (m, 1H, CH), 6.72-7.20 (m, 4H, ArH); IR (KBr) cm -1 : 3390, 2930, 1610, 1590, 1490, 1453...
Embodiment 2
[0030](1) Preparation of compound 1 [(R)-3-m-tolyloxy-1,2-propanediol]
[0031] Dissolve m-cresol (10.8g, 0.1mol) in 120mL of anhydrous methanol, add sodium hydroxide (8.0g, 0.2mol) in batches at room temperature, then add phase transfer catalyst tetrabutylammonium bromide (0.16g, 0.5mmol), drop (R)-3-chloro-1,2-propanediol (25.4g, 0.23mol), dropwise, 55 ~ 60 ° C reaction for 10 hours, the reaction is complete, filtered while hot, and the filtrate was concentrated under reduced pressure, Recrystallization from absolute ethanol and carbon tetrachloride (V:V=5:95) gave 14.9 g of (R)-3-m-tolyloxy-1,2-propanediol as a white solid, yield 81.6%, mp 60-62 °C, [α] D 20 = -9.3 (c 1.0, EtOH).
[0032] (2) Preparation of compound 2 [(S)-4-m-cresyloxymethyl-1,3,2-dioxathiolane-2-oxide]
[0033] Compound 1 (9.1g, 0.05mol) was dissolved in 70mL of dichloromethane, and the mixed solution of thionyl chloride (8.8g, 0.074mol) and 10mL of dichloromethane was added dropwise under temperature...
Embodiment 3
[0037] (1) Preparation of compound 1 [(R)-3-m-tolyloxy-1,2-propanediol]
[0038] Dissolve m-cresol (10.8g, 0.1mol) in 120mL of isopropanol, add sodium hydroxide (6.0g, 0.15mol) in batches at room temperature, and then add phase transfer catalyst tetrabutylammonium bromide (0.16g, 0.5mmol), drop (R)-3-chloro-1,2-propanediol (44.3g, 0.4mol), dropwise, 80 ~ 85 ° C reaction for 3 hours, the reaction is complete, filtered while hot, and the filtrate was concentrated under reduced pressure, Cyclohexane was recrystallized to obtain 15.1 g of white solid (R)-3-m-tolyloxy-1,2-propanediol, yield 82.8%, mp 60-62°C, [α] D 20 = -9.5 (c 1.0, EtOH).
[0039] (2) Preparation of compound 2 [(S)-4-m-cresyloxymethyl-1,3,2-dioxathiolane-2-oxide]
[0040] Compound 1 (9.1g, 0.05mol) was dissolved in 70mL of dichloromethane, and a mixed solution of thionyl chloride (7.7g, 0.065mol) and 10mL of dichloromethane was added dropwise at a temperature of 0-5°C. React at 20-25°C for about 0.5 hours. Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com