Process for recovering dimethyl formyl amine from waste water using ion liquid extracting process
A technology of dimethylformamide and ionic liquid, which is applied in extraction water/sewage treatment, carboxylic acid amide separation/purification, organic chemistry, etc., can solve the problems of organic solvent loss, low recovery rate, secondary pollution, etc. Achieve the effects of reduced loss, no secondary environmental pollution, and short equilibrium time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
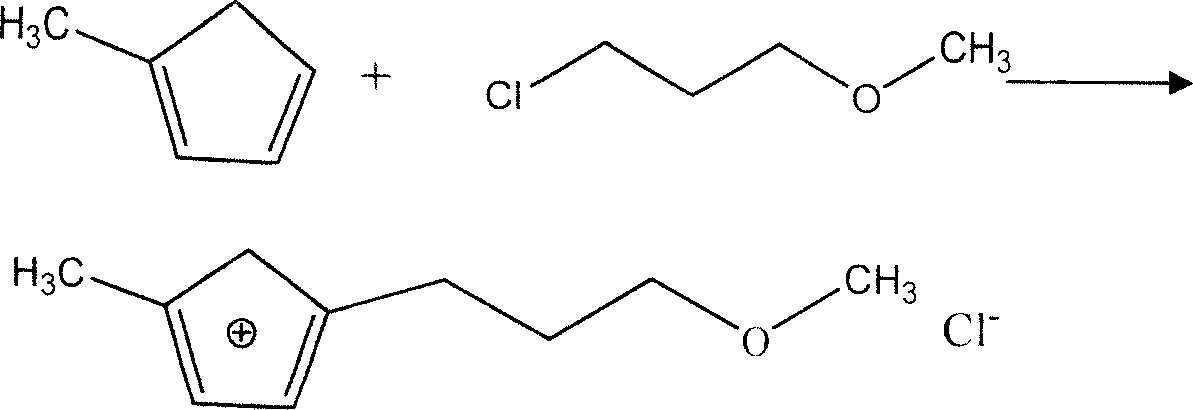
[0025] Embodiment 1: the synthesis of hydrophobic ionic liquid
[0026] Two-step method for synthesizing ionic liquids, first synthesizing 1-methyl-3-propyl methyl ether imidazolium ions, respectively redistilling methyl imidazole and 3-chloropropyl methyl ether before the reaction, and then making the molar ratio 1: Mix the ratio of 1 in a three-necked flask, stir well, heat and reflux in a water bath at 80°C for 24 hours under the protection of nitrogen, wash with ether after cooling to remove unreacted methylimidazole and 3-chloropropyl methyl ether, at 70°C Rotary evaporation 1h obtains 1-methyl-3-propyl methyl ether imidazolium ion; KPF 6 Mix it with the newly synthesized 1-methyl-3-propyl methyl ether imidazolium ion in a molar ratio of 1:1, then carry out the metathesis reaction in a water bath at 80°C for 12 hours, wash with deionized water and dry with anhydrous magnesium sulfate. The impurity was removed by rotary evaporation at 80°C for 10 h, and the desired ionic ...
Embodiment 2
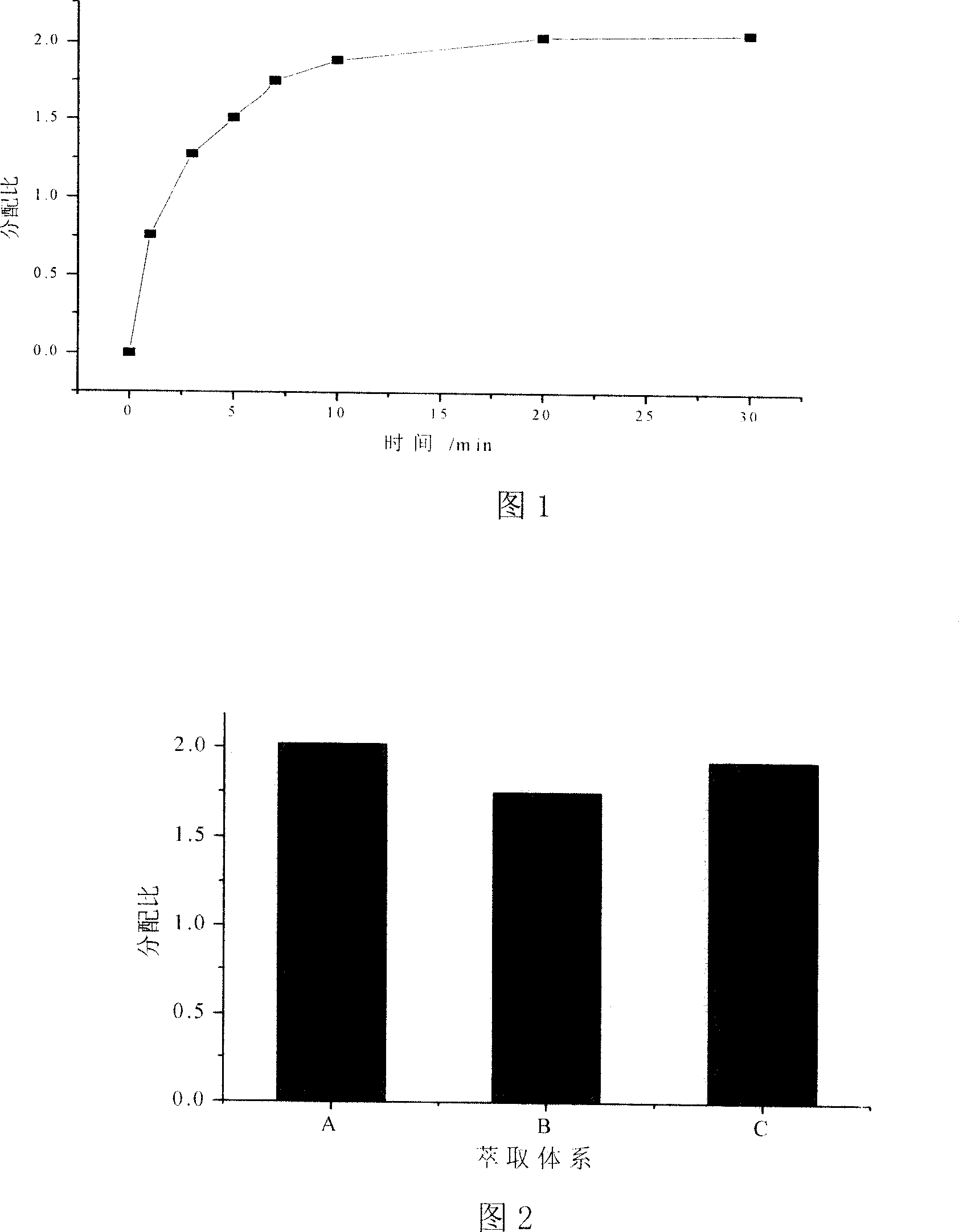
[0027] Example 2: Effect of time on extraction balance
[0028] Using the ionic liquid prepared in Example 1 to extract DMF in the DMF aqueous solution, the specific process is as follows. Accurately measure the DMF aqueous solution that concentration is 8wt%, 30wt%, 50wt%, 70wt% and each 100ml of the ionic liquid made by embodiment 1, the ratio of both by volume 1: 1 or 1: 5, simultaneously once Add it into a mixing tank with stirring (equivalent to single-stage extraction), turn on the stirring to fully mix the two phases, and control the temperature at 20-30°C. Stir for 1, 3, 5, 7, 10, 20, and 30 minutes respectively, then stop stirring, pour into a graduated cylinder, and let stand to separate the phases. After the interface between the two phases is clear, measure the volumes of the aqueous phase and the ionic liquid phase, take the upper aqueous phase with a pipette, and analyze the concentration of DMF contained therein by spectrophotometry. According to the mass bala...
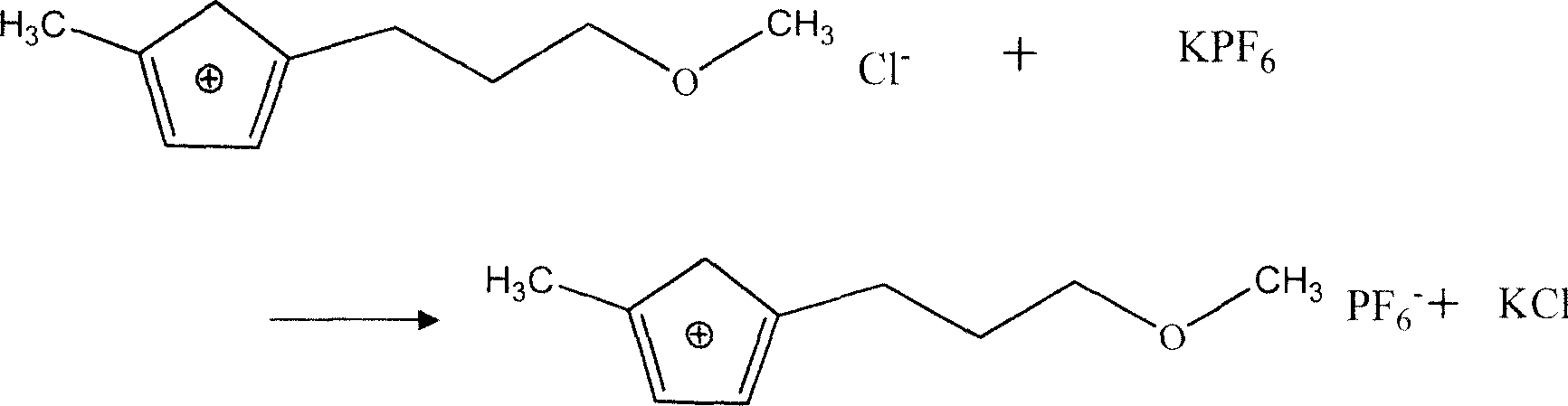
Embodiment 3
[0030] Accurately measure concentration is the DMF aqueous solution of 8wt% and each 100ml of the ionic liquid that is made by embodiment 1, the ratio that both are 1: 1 is added in the mixing tank with agitation (equivalent to single Grade extraction), prepare 3 parts of such extraction systems and mark them as A, B, and C respectively. Slowly add 10wt% H in extraction system B 2 SO 4 , the control pH is 1.0. Add 2 g of NaCl solid to the extraction system C. Start stirring for each extraction system to fully mix the two phases, and control the temperature at 20-30°C. After stirring for 20 minutes, stop stirring, pour into a graduated cylinder, and let stand to separate phases. After the interface between the two phases is clear, measure the volumes of the aqueous phase and the ionic liquid phase, and use a pipette to take the light phase aqueous phase and analyze the concentration of DMF contained therein by spectrophotometry. According to the mass balance of DMF in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com