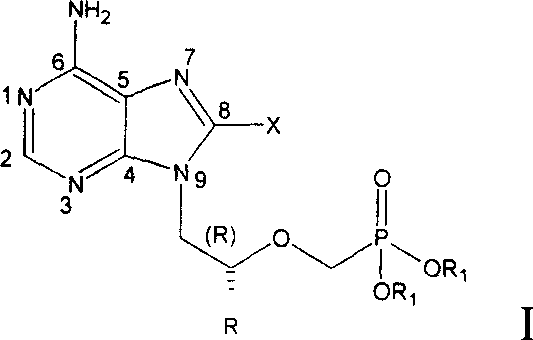
Nucleotide compound in halogenating adenine class, synthetic method, medical appliction
A nucleoside compound, halogenated adenine technology, applied in the field of 8-halogenated adenine nucleoside compounds, can solve problems such as the strong hydrophilic effect of tenofovir, and achieve good application prospects and good fat solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
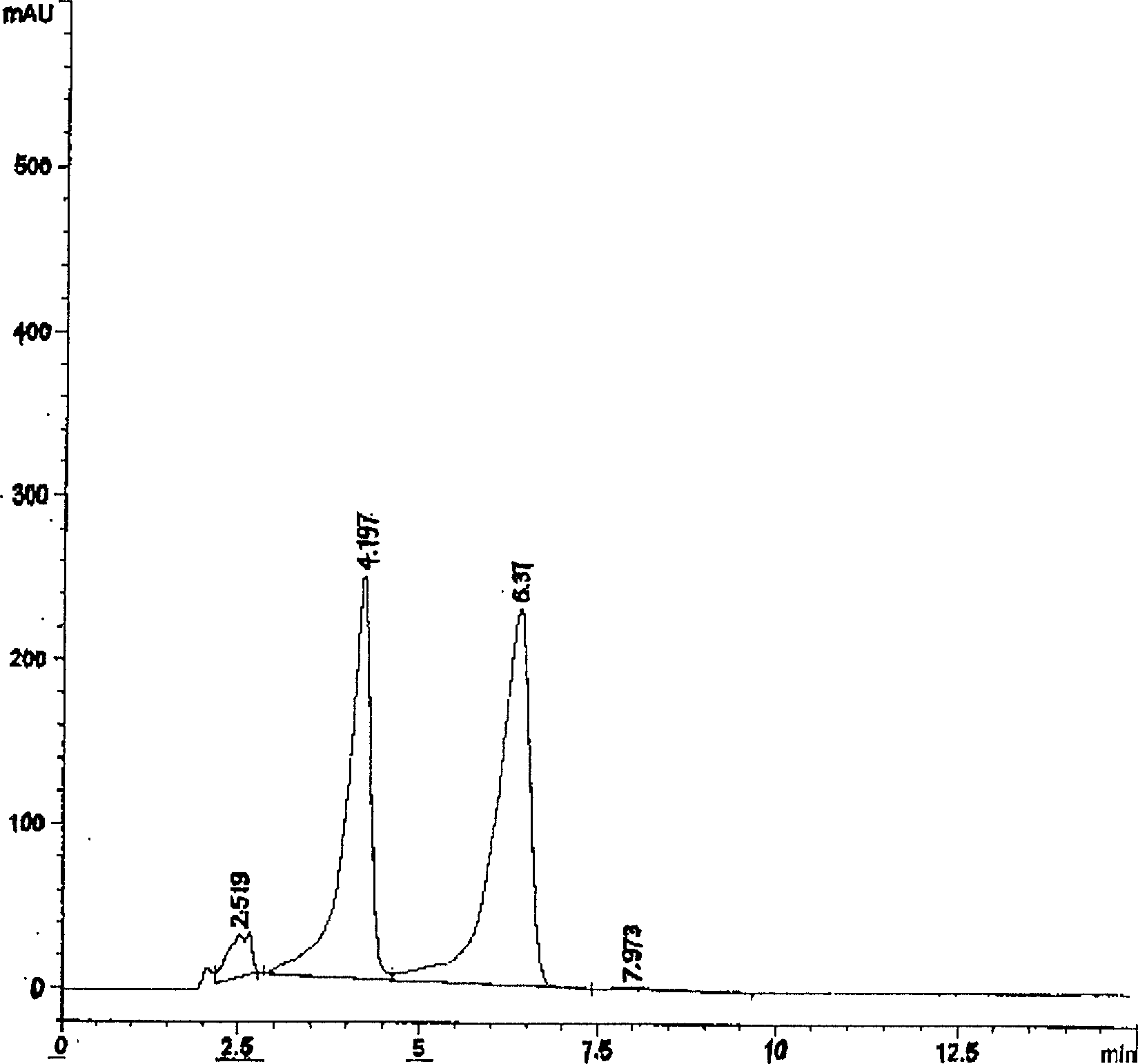
Image
Examples
Embodiment 1
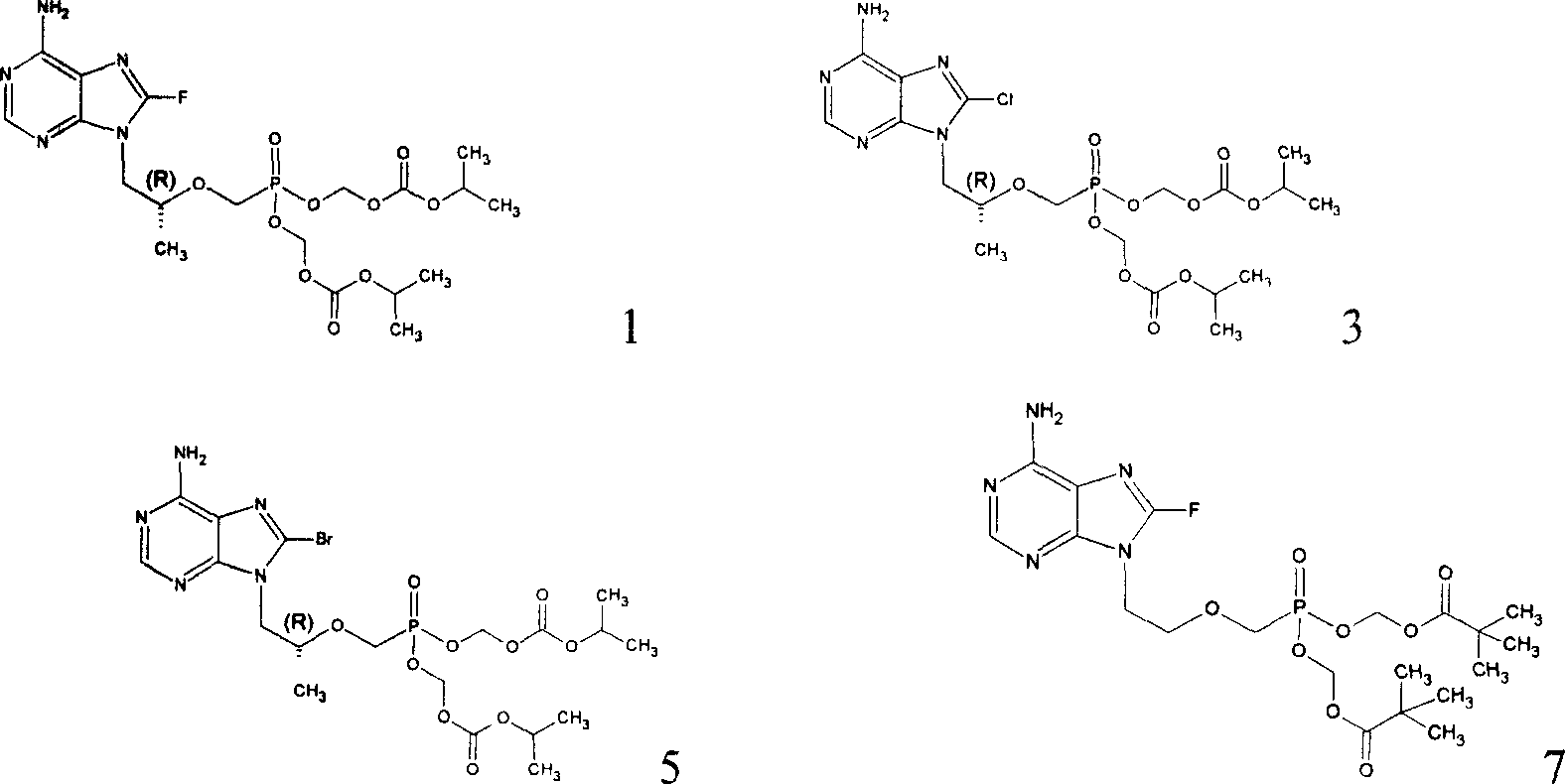
[0044] Example 1.8-bromo-9-(2-dipivaloyloxymethoxyphosphonomethoxy)-ethyl-adenine (compound 11):
[0045]
[0046] Take 9-(2-dipivaloyloxymethoxyphosphonomethoxy)-ethyl-adenine (3.0 g, 6.0 mmol), dissolve it in 30 ml of acetic acid, add sodium acetate (3 g), add Br 2 0.5mL, 9.7mmol). Stir for 2 hours, the raw material point disappears, add ethyl acetate (150ml), water 120ml in turn, add saturated sodium bisulfite (NaHSO 3 ) until the mixture becomes colorless and static, separate the organic phase, adjust the pH of the aqueous phase to 8.0-8.5, extract with ethyl acetate (2 × 100mL), combine the organic phases, and wash with saturated aqueous sodium chloride, anhydrous sodium sulfate After drying, the organic solvent was distilled off to obtain 3.10 g of the title compound. mp.99℃,FAB HRMS[M+H + ]calcd for C 20 h 32 BrN 5 o 8 P 581.3740.found 581.3747.
Embodiment 2
[0047] Example 2.8-bromo-9-(2-(R)-diisopropoxycarbonyloxymethoxyphosphonomethoxy)-propyladenine-fumaric acid (compound 6):
[0048]
[0049] (1) 8-bromo-9-(2-(R)-diisopropoxycarbonyloxymethoxyphosphonomethoxy)-propyladenine (compound 5):
[0050] Take 9-(2-(R)-diisopropoxycarbonyloxymethoxyphosphonomethoxy)-propyladenine (3.3 g, 6.3 mmol), dissolve it in 30 ml of acetic acid, add sodium acetate (1.0 g ), add Br 2 (0.50mL, 9.7mmol). Stir for 2 hours, the raw material point disappears, add ethyl acetate (180ml), water (150ml) in turn, add saturated sodium bisulfite (NaHSO 3 ) until the mixture becomes colorless and static, separate the organic phase, adjust the pH of the aqueous phase to 8.0-8.5, extract with ethyl acetate (280mL), combine the organic phases, wash with saturated aqueous sodium chloride, and dry over anhydrous sodium sulfate. The organic solvent was distilled off to obtain viscous compound 5. 1 H NMR (CD 3 OD) δppm8.19(s, 1H), 6.75(s, 2H, CH 2 ), 5.56-5.4...
Embodiment 3
[0053] Example 3.8-chloro-9-(2-(R)-diisopropoxycarbonyloxymethoxyphosphonomethoxy)-propyladenine-fumaric acid
[0054] (1) 8-chloro-9-(2-(R)-diisopropoxycarbonyloxymethoxyphosphonomethoxy)-propyladenine
[0055] Take 9-(2-(R)-diisopropoxycarbonyloxymethoxyphosphonomethoxy)-propyladenine (3.0 g), dissolve it in 50 ml of acetic acid, add sodium acetate (3.0 g), and stir Chlorine gas was introduced at the bottom, and stirred for 2 hours. When TLC detected that the raw material point disappeared, ethyl acetate (100ml) and water 150ml were added successively, and 5% sodium bisulfite (NaHSO4) was added under stirring. 3 ) until the mixture is detected by starch potassium iodide test paper and does not turn blue, static layering, separating the organic phase, adjusting the pH of the aqueous phase to 8.0-8.5, extracting with ethyl acetate (2 × 60mL), combining the organic phases, and using saturated sodium chloride After washing with aqueous solution and drying over anhydrous sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com