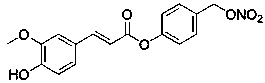
Application of gastrodine derivative
A derivative, gastrodin technology, applied in the application field of gastrodin derivatives, can solve the problems of toxic side effects and low bioavailability, and achieve the effects of strong stability, short synthesis steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
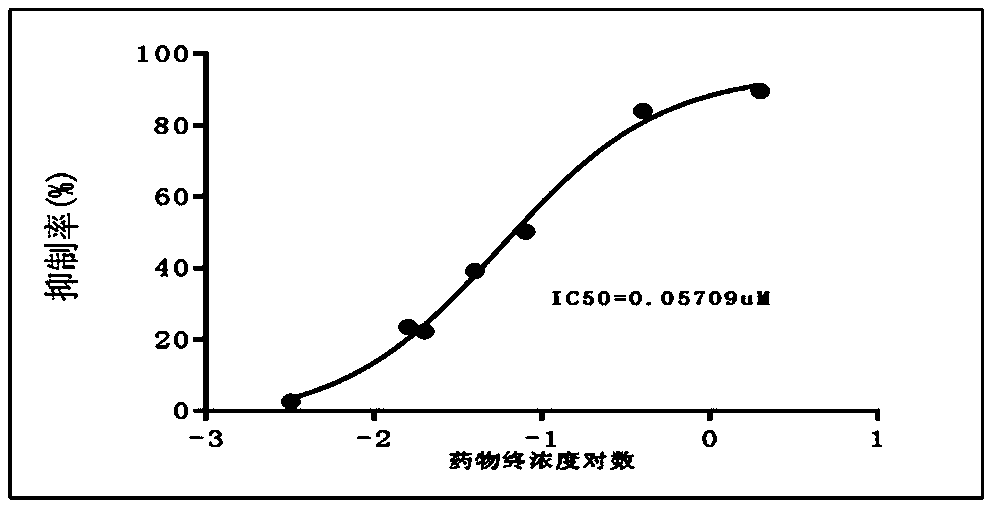
[0025] Example 1 Inhibition of cholinesterase
[0026] experiment material:
[0027] Test sample: KPC-3000207 (namely the above-mentioned gastrodin derivative), white powder, batch number JT20161014, content >98%;
[0028] Reagents: Acetylcholinesterase (CAS: 9000-81-1), Sigma, batch number SLBK4849V; Acetylcholine iodide (CAS: 2260-50-6) purity ≥ 98%, Sigma, batch number BCBL0098V; DTNB, 5,5-dithiobis( 2-nitrobenzoic acid) (5,5'-Dithiobis (2-nitrobenzoic acid)) (CAS: 69-78-3), Sigma, batch number SHBD2937V; Tris-Base, Purity≥99.9%, Amresco product, Exp: 201512; DMSO, Dimethylsuifoxide, sigma, batch number BCBM7089V; Concentrated hydrochloric acid, Yunnan Shandian Pharmaceutical Co., Ltd., batch number 20090402; NaOH, Shanghai Abby Chemical Reagent Co., Ltd., batch number 20110223; NaHCO3, Beijing Lingyun Building Materials Chemical Co., Ltd., batch number 20090805; Ultrapure water, ready to use, was prepared by a Milli-Q ultrapure water machine (Merch Millipore, Germany). ...
Embodiment 2
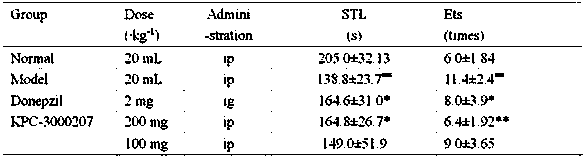
[0039] Example 2 In vivo efficacy verification
[0040]Establish rat and mouse cognitive impairment models to verify the pharmacological effects of gastrodin derivatives in vivo.
[0041] experiment material:
[0042] YLS-17B dark shuttle recorder, Shandong Jinan Yiyan Technology Development Co., Ltd.; PowerWaveXS2 full-wavelength microplate reader, BioTek; Q55-200 ultrasonic breaker, Gene Company Limited; AC211S electronic analytical balance, Sartorius Company; LT2000B electronic balance , Changshu Tianliang Instrument Co., Ltd.; DHG-9245A electric constant temperature blast drying oven, Shanghai Yiheng Scientific Instrument Co., Ltd.; Eppendorf series of micro pipettes with various ranges; beaker, measuring cylinder, scissors, ophthalmic curved forceps, hemostat , arterial clips, sutures and other consumables are all made in China.
[0043] Experimental animals:
[0044] SPF-grade male ICR mice (4-5 weeks old, 20-25 g) were provided by the Experimental Animal Laboratory o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com