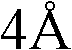Method for synthesizing and purifying fat-soluble rosmarinic acid
A technology of rosmarinic acid ester and rosmarinic acid, which is applied in the field of organic acid ester production in the organic chemical industry, can solve problems such as limited application range, and achieve expanded application range, strong oxidation resistance, and good fat solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
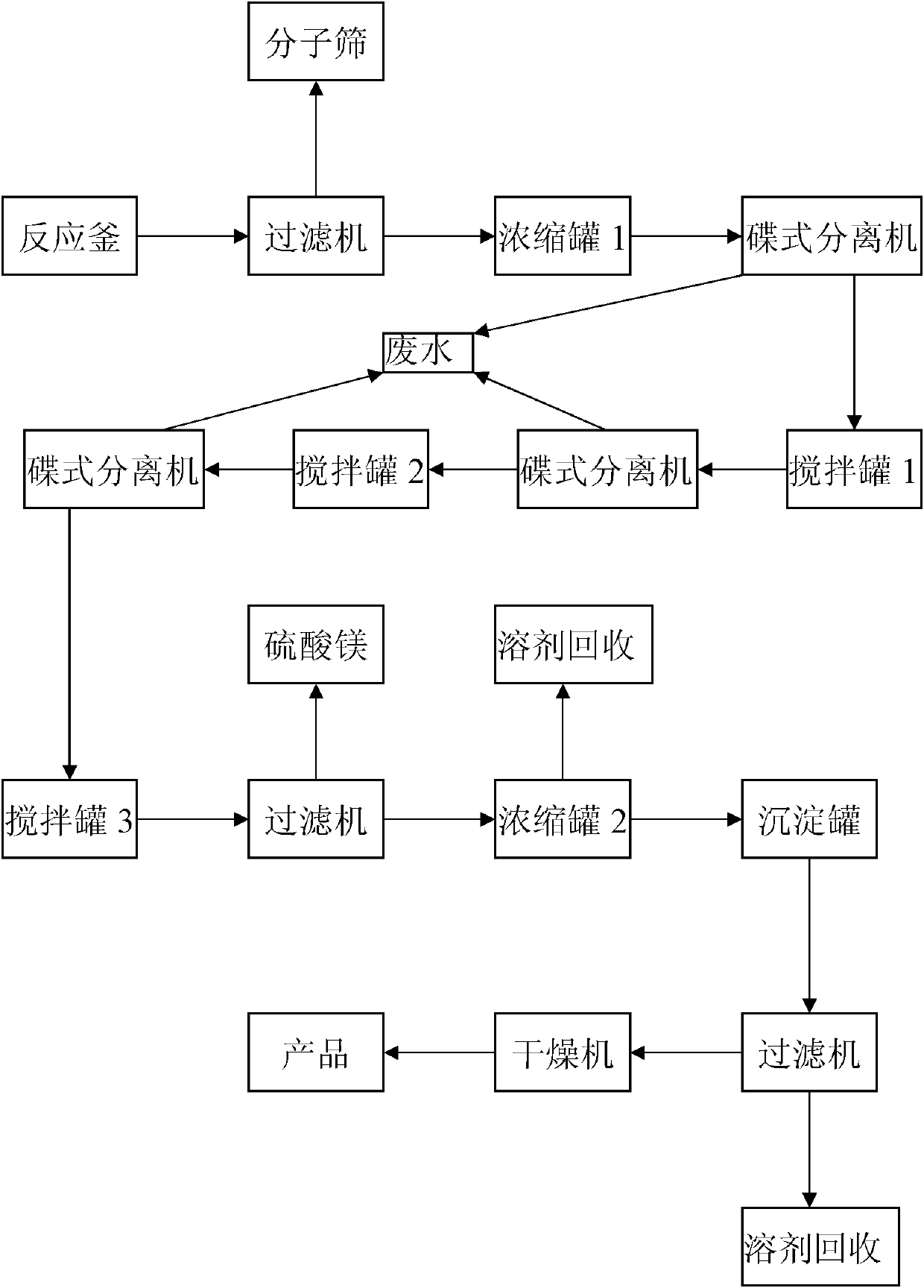
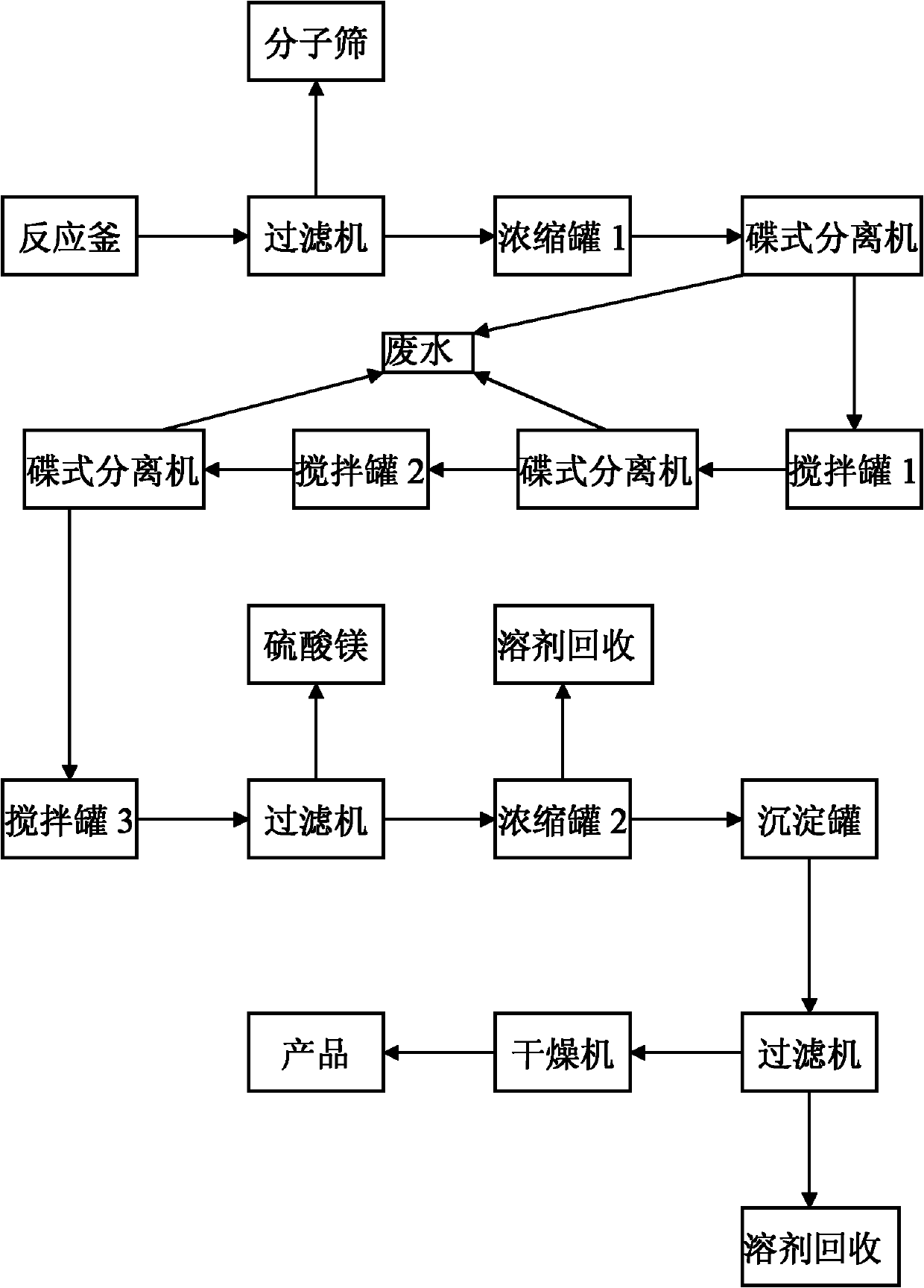
[0019] Such as figure 1 As shown, in the present embodiment, 1Kg rosmarinic acid is added to a 200L reactor (equipped with a reflux device), and 100L of 1,4-dioxane, 0.5Kg of p-toluenesulfonic acid, 766g of dodecanol are added ,500 g Molecular sieves are heated to the boiling point of 1,4-dioxane, and the 1,4-dioxane is slowly refluxed, and the reaction time is 30 hours.
[0020] After the reaction, remove the molecular sieve through a filter, collect the filtrate in a 200L concentration tank 1, concentrate in vacuo at 53°C and recover the solvent 1,4-dioxane until no liquid flows out; then slowly add 100L of acetic acid to the concentration tank Dissolve the ethyl ester, and wash the organic phase with 30L of deionized water, and separate the water phase and the ethyl acetate phase with a disc separator; the water phase is decontaminated, and the ethyl acetate phase is transferred to the 200L mixing tank 1, and 30L is added to the mixing tank 1 Wash with deionized water; s...
Embodiment 2
[0023] Such as figure 1 As shown, in the present embodiment, 1Kg rosmarinic acid is added to a 200L reactor (equipped with a reflux device), and 100L of 1,4-dioxane, 0.5Kg of p-toluenesulfonic acid, and 883g of tetradecyl alcohol are added ,500 g Molecular sieves are heated to the boiling point of 1,4-dioxane, and the 1,4-dioxane is slowly refluxed, and the reaction time is 36 hours.
[0024] After the reaction, remove the molecular sieve through a filter, collect the filtrate in a 200L concentration tank 1, concentrate in vacuo at 53°C and recover the solvent 1,4-dioxane until no liquid flows out; then slowly add 100L of acetic acid to the concentration tank Dissolve the ethyl ester, and wash the organic phase with 30L of deionized water, and separate the water phase and the ethyl acetate phase with a disc separator; the water phase is decontaminated, and the ethyl acetate phase is transferred to the 200L mixing tank 1, and 30L is added to the mixing tank 1 Wash with deion...
Embodiment 3
[0027] Add 1Kg rosmarinic acid to a 200L reactor (equipped with a reflux device), and add 100L of 1,4-dioxane, 0.6Kg of p-toluenesulfonic acid, 906g of cetyl alcohol, 600g Molecular sieves are heated to the boiling point of 1,4-dioxane, and the 1,4-dioxane is slowly refluxed, and the reaction time is 38 hours.
[0028] After the reaction is over, remove the molecular sieves through a filter, collect the filtrate in a 200L concentration tank 1, concentrate in vacuo at 55°C and recycle the solvent 1,4-dioxane until no liquid flows out; then slowly add 120L of tris Methyl chloride was dissolved, and the organic phase was washed with 35L of deionized water, and the water phase and the chloroform phase were separated with a disc separator; the water phase was decontaminated, and the chloroform phase was transferred to the 200L mixing tank 1, and 35L was added to the mixing tank 1 Wash with deionized water; separate the water phase and the chloroform phase with a disc separator aga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com