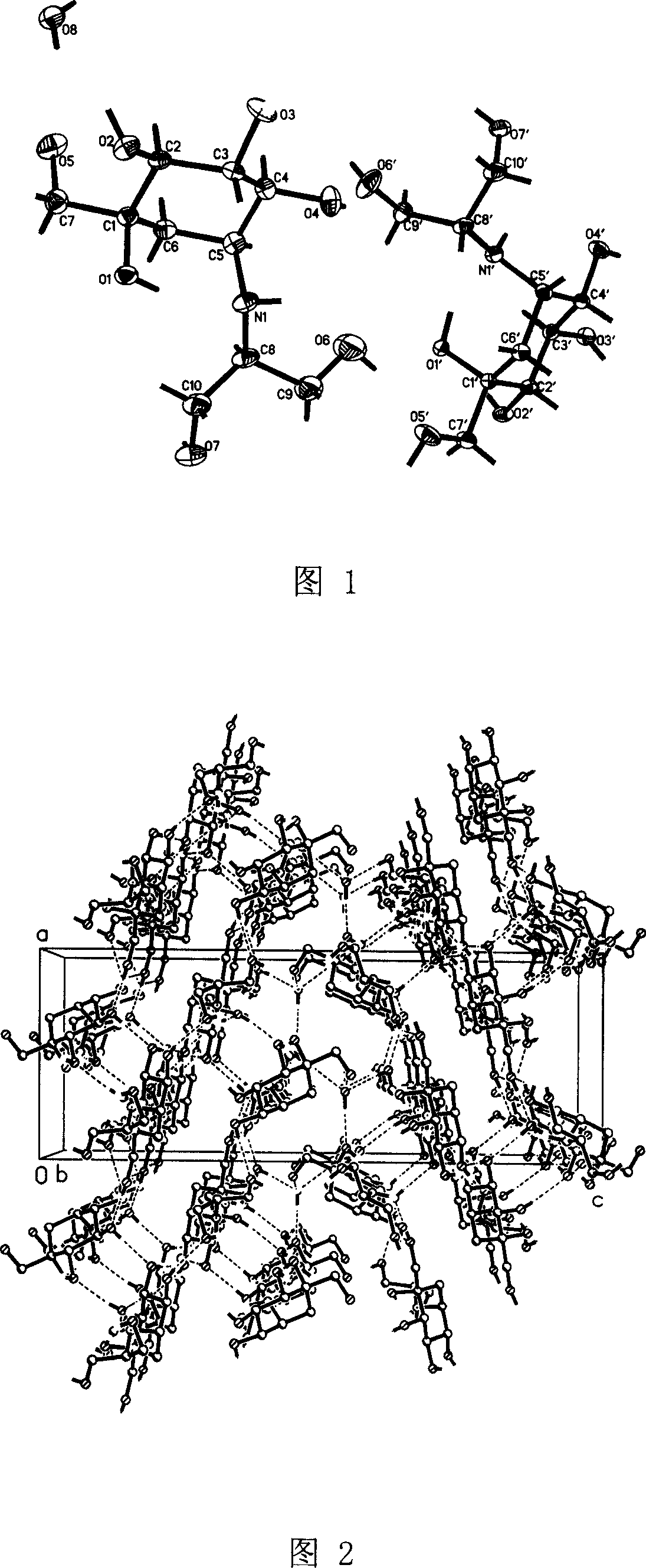
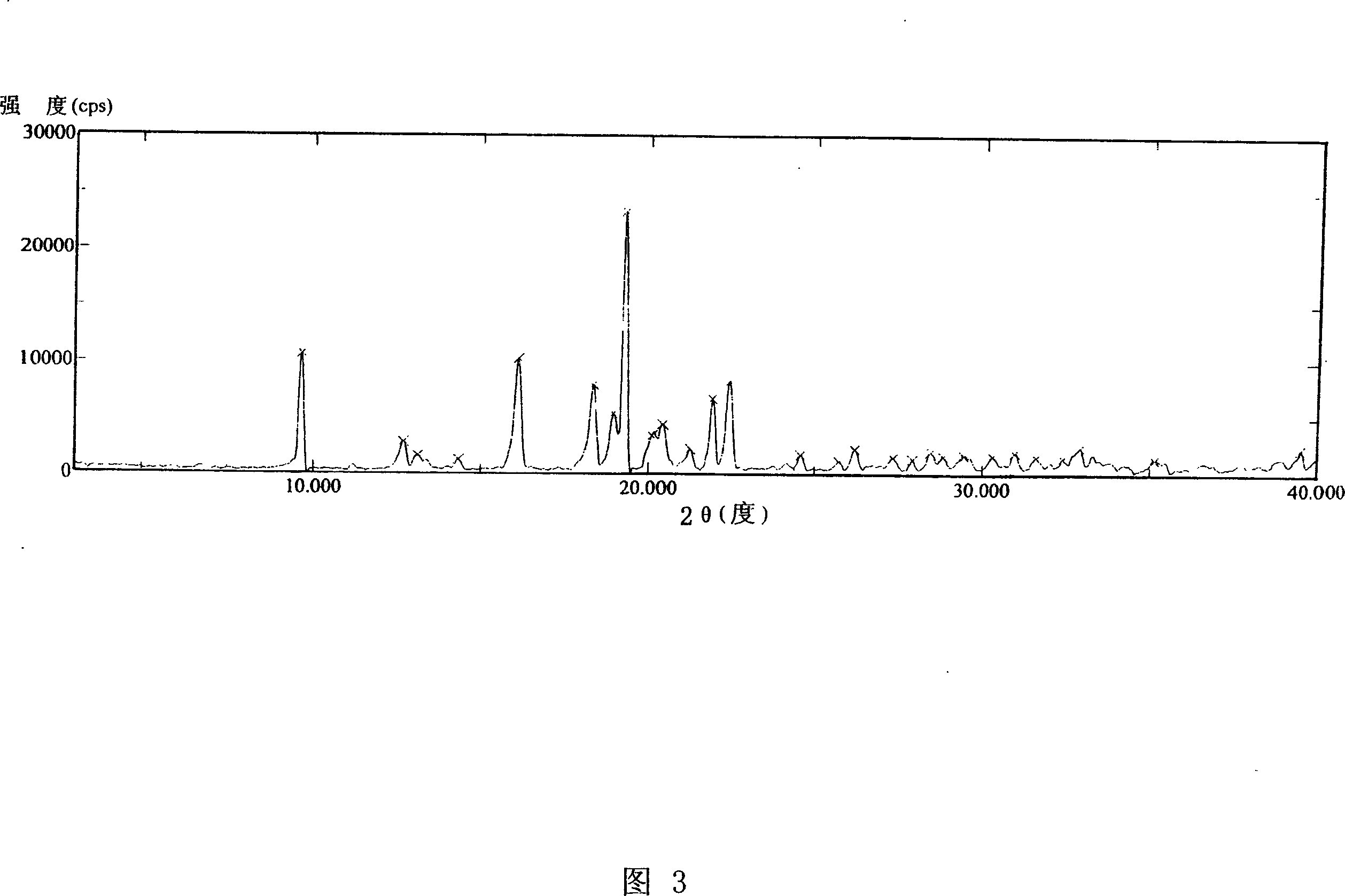
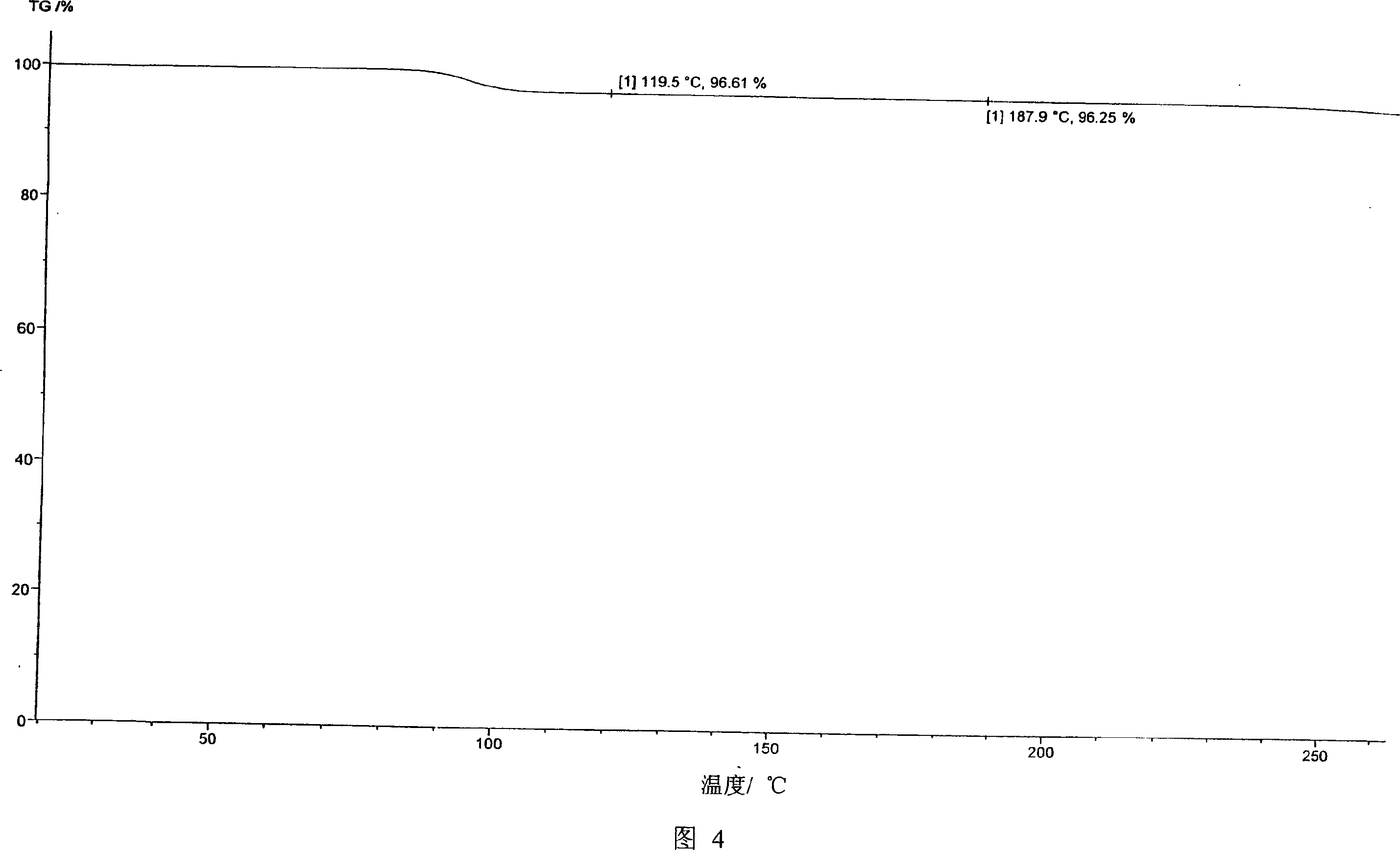
Voglibose semi-hydrated crystal, its preparation method and its uses in medicament formulation
A technology for voglibose and hemihydrate, which is applied in the field of preparation of voglibose hemihydrate crystals, and can solve the problems of insufficient crystal precipitation, low yield, and increased processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 (1S)-(1(hydroxy), 2,4,5 / 1,3)-5-[(2-hydroxy-1-(hydroxymethyl)ethyl)amino]-1-carbon-hydroxy Preparation of methyl-1,2,3,4-cyclohexanetetraol hemihydrate crystals (voglibose hemihydrate crystals)
[0087] Dissolve tetrabenzyl voglibose (300.0g, 0.48mol) in 90% formic acid / methanol (1:19, 6L), add palladium black (60g), react at room temperature for 12 hours under nitrogen protection, and filter Wash with methanol / water (1:1) 2L, concentrate the filtrate, absorb the residue with strong acid ion exchange resin (5L), wash with water, and then eluted with 0.5N ammonia water. After the eluent is evaporated to remove water, add no 2L of water and ethanol, stirring, filtering the precipitated light gray crystals, and vacuum drying for 12 hours to obtain 110 g of crude voglibose.
[0088] Dissolve 100g of the above crude voglibose in 200g of hot pure water, add 200g of ethanol, 10g of activated carbon, heat at 60°C for 30 minutes to decolor, filter, add 500g of ethanol at 60...
Embodiment 2
[0090] Example 2 Preparation of voglibose hemihydrate crystals
[0091] Dissolve 100g of the crude voglibose (the preparation method is the same as in Example 1) in 100g of hot pure water, add 100g of methanol, 10g of activated carbon, heat at 60°C for 30 minutes to decolorize, filter, add 1000g of methanol at 50°C to the filtrate, and stir. The crystals were precipitated, cooled to room temperature naturally, filtered, and dried under vacuum at 40°C for 12 hours to obtain 85 g of voglibose hemihydrate crystals.
Embodiment 3
[0092] Example 3 Preparation of voglibose hemihydrate crystals
[0093]Dissolve 100g of the crude voglibose (the preparation method is the same as in Example 1) in 300g of hot pure water, add 300g of ethanol, 10g of activated carbon, heat at 60°C for 30 minutes to decolorize, filter, and add 300g of isopropanol at 70°C to the filtrate. Crystals were precipitated under stirring, cooled to room temperature naturally, filtered, and vacuum dried at 40°C for 48 hours to obtain 90 g of voglibose hemihydrate crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com