Human horny cell growth factor-2 mutant and its preparation method
A technology of keratinocytes and growth factors, applied in the field of genetic engineering, can solve problems such as failure, and achieve the effects of simplified operation, simplified purification process, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
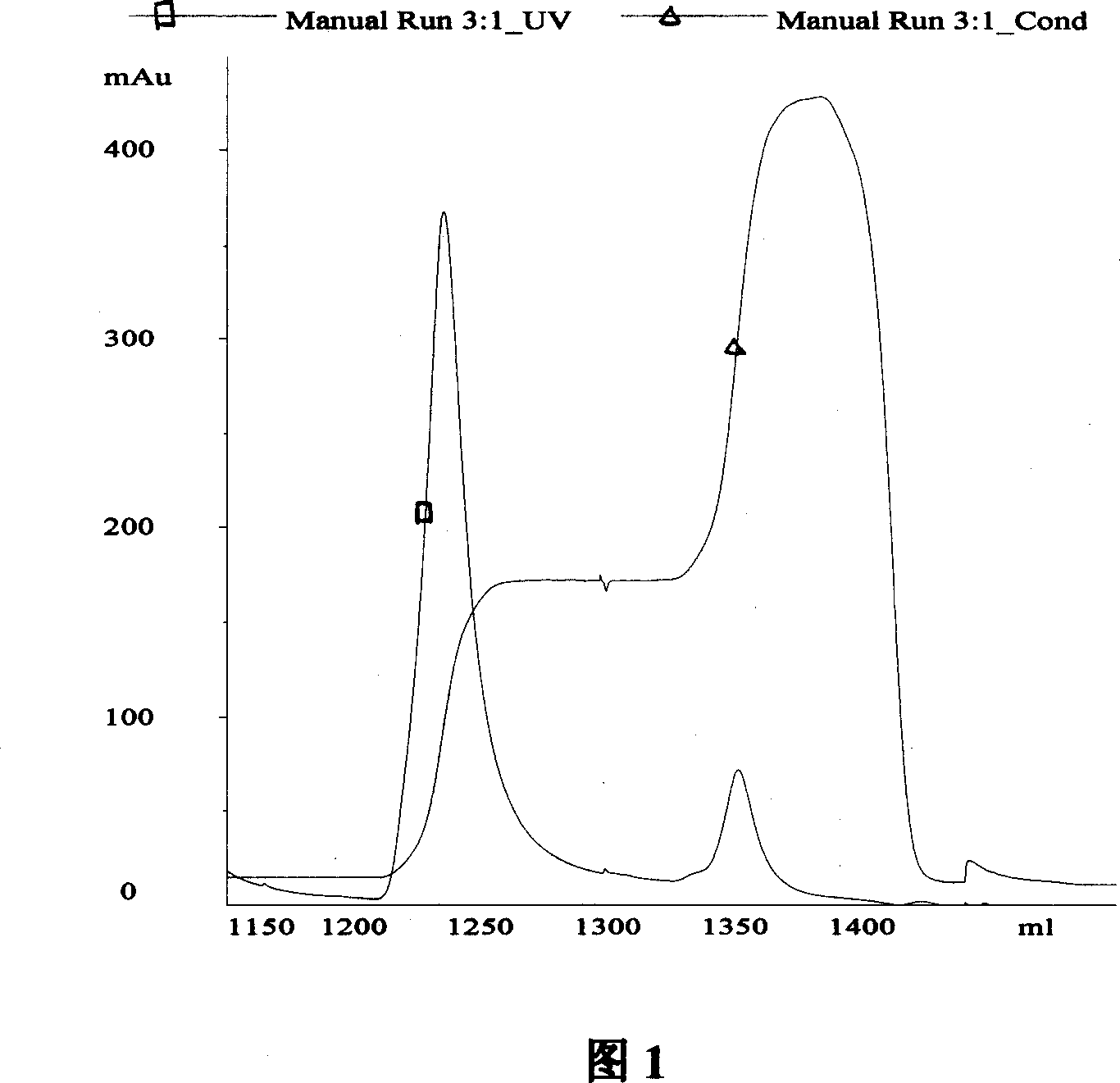
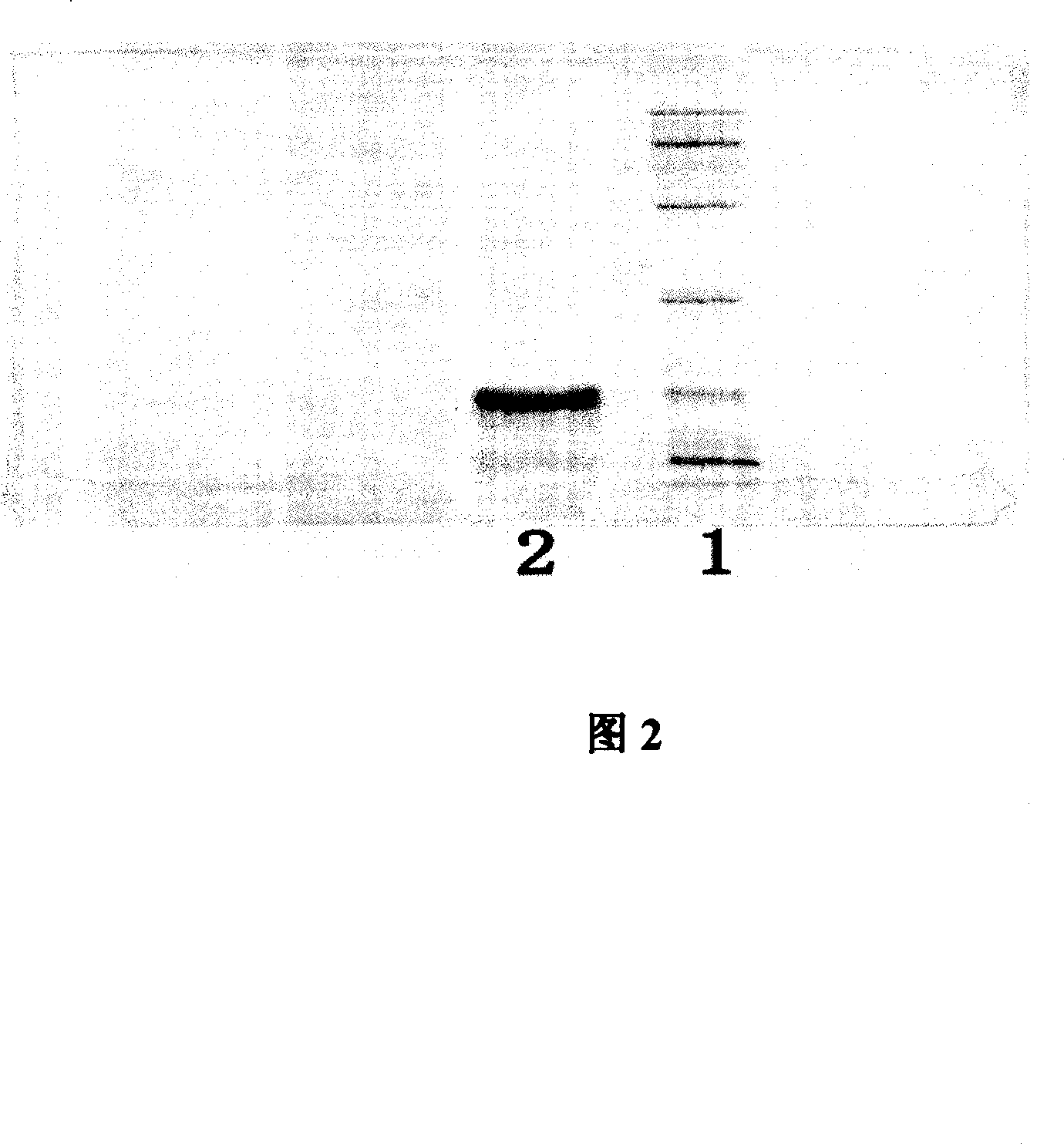
Image
Examples
Embodiment 1
[0058] Construction of different KGF-2 mutant expression plasmids
[0059] The mature peptide of natural human KGF-2 has 171 amino acid residues. Experiments show that removing the first 26 amino acid residues at the N-terminus of KGF-2 does not affect the binding of KGF-2 to its receptor. The removal of 26 amino acid residues of KGF The complex of -2 and its receptor was also crystallized and the three-dimensional structure was resolved. In order to efficiently express KGF-2 in E. coli, it may be considered to remove part of the N-terminal amino acid residues that do not affect its activity. The nucleotide sequence encoding human KGF-2 C-terminal 145-171 amino acid residues was chemically synthesized. Due to chemical synthesis, in order to express KGF-2 in E. coli to the greatest extent, the preferred codons of E. coli are used for synthesis. In order to realize natural expression and simplify the subsequent processing technology, a start codon ATG was added to the 5'end, and a s...
Embodiment 2
[0061] Expression analysis to pick the best mutant
[0062] The constructed KGF-2 mutant was transformed into E. coli BL21(DE3) competent cells, and the transformants were screened by antibiotics. Select well-separated colonies to inoculate LB medium containing appropriate antibiotics, add IPTG to 1 mM and culture for 4 hours to induce KGF-2 mutant expression. SDS polyacrylamide gel electrophoresis was used to separate the total bacterial protein, stained with Coomassie Brilliant Blue R250, and the percentage of KGF-2 expressed in the total protein was analyzed by gel scanning. By comparison, we selected a mutant with the first Q removed from the N-terminus. If Q is removed, the amino acid residue after M is exactly A. This is very consistent with the N-terminal rule: the second amino acid residue being Q will make the expressed protein unstable, while the second amino acid residue being A will greatly stabilize the expressed protein. The selected DNA sequence and its encoded prot...
Embodiment 3
[0086] Induced expression of recombinant KGF-2 mutant
[0087] The engineered bacteria selected in Example 2 were inoculated into LB medium containing corresponding antibiotics, and cultured with shaking at 100-300 times / min for 12-14 hours. Inoculate the LB medium containing the same antibiotic at a ratio of 1:100-1:20, and continue to culture for 3-6 hours, the culture temperature is 20-37°C, and the shaking speed is 100-300 times / min. Add IPTG to a final concentration of 0.1-2mM, and continue to incubate for 3-4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com