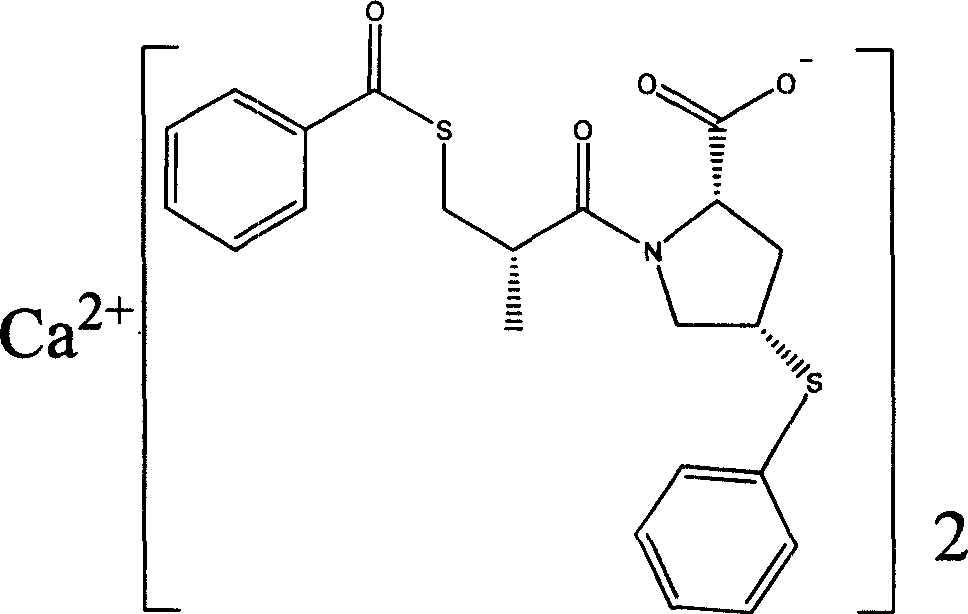
Method of preparing zofenopril calcium
A technology of zofenopril calcium and phenylthio group, which is applied in the field of preparation of zofenopril calcium, can solve the problems of high raw material cost, increased product cost, complicated operation and the like, and achieves the effects of easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
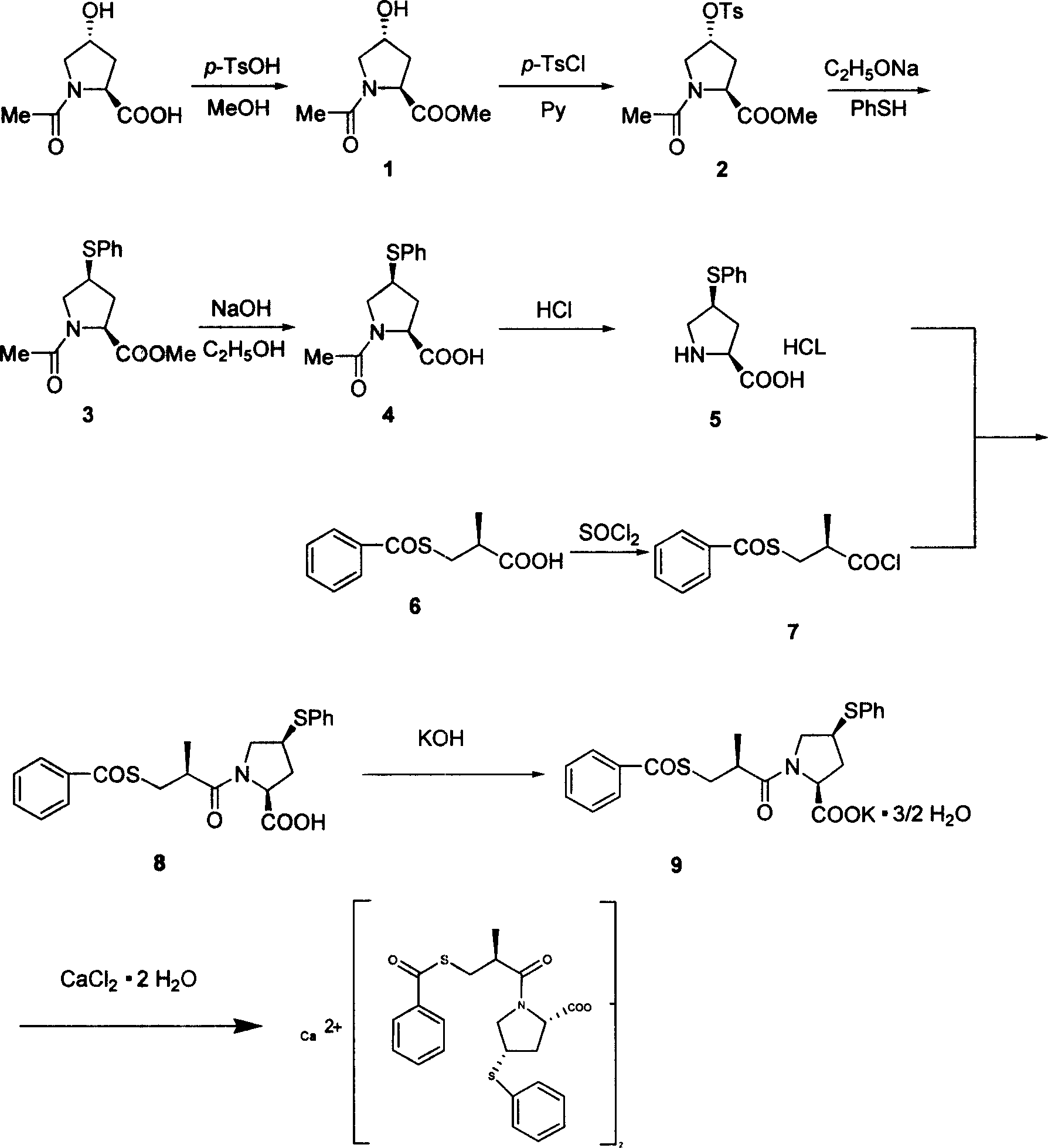
[0025] (1) Take 84g of N-acetyl-hydroxyproline and 150ml of anhydrous methanol, stir to dissolve, 16.58g of p-toluenesulfonic acid, react, add solid sodium bicarbonate to adjust pH=7, filter the filtrate and add anhydrous sulfuric acid Sodium-dried, filtered, concentrated and distilled to remove the solvent to obtain 85 g of a light yellow oily substance, namely N-acetyl-L-hydroxyproline methyl ester (intermediate (1)), with a yield of 93.6%.
[0026] (2) Take 84.2 g of intermediate (1) and 150 ml of pyridine, add 102.6 g of p-toluenesulfonyl chloride, stir to precipitate a white solid, add 50 ml of water to dissolve the solid, extract with 75 ml of dichloromethane × 3 times, combine the organic layers, Wash with water twice in turn. The organic layer was dried and filtered, and the solvent was evaporated to obtain 107.6 g of a yellow-brown oily substance, that is, N-acetyl-trans-4-p-toluenesulfonyl-L-hydroxyproline methyl ester (intermediate (2)), and the yield was 70.0%.
...
Embodiment 2
[0035] (1) Take 840g of N-acetyl-hydroxylamine and 1.5L of anhydrous methanol, stir to dissolve, add 165.8g of p-toluenesulfonic acid, stir at room temperature for 48h, add sodium bicarbonate to adjust pH=7, filter, and then Add anhydrous sodium sulfate to dry, filter, and distill off the solvent to obtain 866 g of light yellow oily intermediate (1), with a yield of 96%.
[0036] (2) Take 842g of intermediate (1) and 1500ml of pyridine, add 1026g of p-toluenesulfonyl chloride, stir to have a white solid precipitate out, add 500ml of water to dissolve the solid, extract with 750ml of dichloromethane × 3 times, combine the organic layers, and use Washed twice with water; the organic layer was dried and filtered with anhydrous sodium sulfate, and the solvent was evaporated to obtain 1086 g of yellow-brown oil intermediate (2), with a yield of 70.7%.
[0037] (3) Get 1500ml of absolute ethanol and add it to a dry reaction vessel, slowly add 215g of chopped metal sodium in batches,...
Embodiment 3
[0045] (1) Take 840g of N-acetyl-hydroxyproline and 1.5L of anhydrous methanol, stir to dissolve, add 165.8g of p-toluenesulfonic acid, stir at room temperature for 48h, add sodium bicarbonate to adjust the pH to 7, filter, and refill the filtrate Add anhydrous sodium sulfate to dry, filter, and distill off the solvent to obtain 848 g of light yellow oil intermediate 1) with a yield of 94%.
[0046] (2) Take 842g of intermediate (1) and 1500ml of pyridine, add 1026g of p-toluenesulfonyl chloride, stir to have a white solid precipitate out, add 500ml of water to dissolve the solid, extract with 750ml of dichloromethane × 3 times, combine the organic layers, and use Wash twice. The organic layer was dried and filtered with anhydrous sodium sulfate, and the solvent was evaporated to obtain 1109 g of yellow-brown oily intermediate (2). The yield was 72.2%.
[0047] (3) Take 1500ml of absolute ethanol and add it to a dry reaction vessel, slowly add 215g of chopped metal sodium in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com